This set of Pulp and Paper Multiple Choice Questions and Answers (MCQs) focuses on “Sulphite Liquor Analysis”.
1. Sulfite pulping liquors could be titrated with NaOH to each endpoint to determine the free SO2 and combined SO2.
a) True
b) False
View Answer
Explanation: Sulfite pulping liquors could be titrated with NaOH to each endpoint to calc. the free SO2 and combined SO2.
2. _________ developed a method where, under acidic conditions, all of the SO2 is converted to SO4-2 by periodate ion.
a) Kraft
b) Kennedy
c) Layman
d) Palmrose
View Answer
Explanation: Palmrose (1935) developed a method where, under acidic conditions, all of the SO2 is converted to SO4-2 by periodate ion. Periodate thus measures the total SO2.
3. Fill in the blank.
KIO3 + 3H2SO3andrarr; ___________+ 3H2SO4
a) 6I–
b) 2KI
c) 3SO4-2
d) KI
View Answer
Explanation: I of KIO3 is reduced from +5 to -1 while each sulfur of SO4-2is oxidized from +4 to +6. The equivalent weight of KIO3 is 1/6 the molecular weight, and the equivalent weight of H2CO3 one half the molecular weight. These reactions are actually fairly slow and the endpoint would easily be overrun. Small amounts of KI (from the indicator solution), however, allow the following two reactions, which are rapid.
4. Fill in the blank.
IO– + 3H2SO3 + 5I–
andrarr; ____________ + 3I2 + 3H2O
a) 6I–
b) 2KI
c) 3SO4-2
d) KI
View Answer
Explanation: I of KIO3 is reduced from +5 to -1 while each sulfur of SO4-2is oxidized from +4 to +6. The equivalent weight of KIO3 is 1/6 the molecular weight, and the equivalent weight of H2CO3 one half the molecular weight. These reactions are actually fairly slow and the endpoint would easily be overrun. Small amounts of KI (from the indicator solution), however, allow the following two reactions, which are rapid.
5. Fill in the blank.
2KIO3 + 3(H2SO3)–andrarr;___________ + 3H2SO4 + 3SO42-
a) 6I–
b) 2KI
c) 3SO4-2
d) KI
View Answer
Explanation: I of KIO3 is reduced from +5 to -1 while each sulfur of SO4-2is oxidized from +4 to +6. The equivalent weight of KIO3 is 1/6 the molecular weight, and the equivalent weight of H2CO3 one half the molecular weight. These reactions are actually fairly slow and the endpoint would easily be overrun. Small amounts of KI (from the indicator solution), however, allow the following two reactions, which are rapid.
6. Fill in the blank.
3SO42- + 3I2 + 3H2O + 3H2SO3andrarr; __________+ 6H2SO4
a) 6I–
b) 2KI
c) 3SO4-2
d) KI
View Answer
Explanation: I of KIO3 is reduced from +5 to -1 while each sulfur of SO4-2is oxidized from +4 to +6. The equivalent weight of KIO3 is 1/6 the molecular weight, and the equivalent weight of H2CO3 one half the molecular weight. These reactions are actually fairly slow and the endpoint would easily be overrun. Small amounts of KI (from the indicator solution), however, allow the following two reactions, which are rapid.
7. What is the name of the compound?
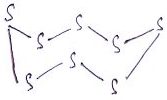
a) Catenasulfur
b) Cyclooctasulfur
c) Sulphite liquor
d) Muthmann’s sulfur I
View Answer
Explanation: Octasulfur is an inorganic chemical, S8 is a yellow solid, and is odourless and tasteless. It’s the most common allotrope of sulfur and a major industrial chemical that occurs widely in nature.
8. What is the name of the compound?
![]()
a) Catenasulfur
b) Cyclooctasulfur
c) Sulphite liquor
d) Muthmann’s sulfur I
View Answer
Explanation: This the quenched product of sulfur melts above 160 °Celcius at this point the properties of the liq. melt change remarkably, e.g. large inc. in viscosity. Its form changes from an initial plastic form steadily to a glassy form. It contains a mixture of catena-sulfur forms mixed with cyclo-forms.
9. _________ sulfur occurs in many complex forms. The chemistry of elemental sulfur presented is a simplification of its complex chemistry.
a) Nitrous
b) Carbonated
c) Elemental
d) Catena
View Answer
Explanation: Elemental sulfur occurs in many complex forms. The chemistry of elemental sulfur presented is a simplification of its complex chemistry but will be important to explain its properties.
10. Above _________ no sulfur trioxide is produced; however, some might be produced in the process of cooling the gases.
a) 1000
b) 550
c) 1500
d) 2000
View Answer
Explanation: Above 1000°Celcius no sulfur trioxide is produced; however, some might be produced in the process of cooling the gases. SO3, which produces sulfuric acid upon reaction with water, is very undesirable in pulping reactions and is removed during the cooling/scrubbing process by opposite current flow of water and SO2.
Sanfoundry Global Education & Learning Series – Pulp and Paper.
To practice all areas of Pulp and Paper, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
