This set of Pulp and Paper Multiple Choice Questions and Answers (MCQs) focuses on “Forms of Monosaccharides”.
1. Stereochemistry is introduced at the aldehyde C atom, there are 2 possible products, which are known as anomers.
a) Monomers
b) Anomers
c) Monosaccharies
d) Glycosidic
View Answer
Explanation: It becomes asymmetric, there are 2 possible products, which are known as anomers. For the pyranose or furanose stereochemistry is formed at the aldehyde.
2. The C atom of the aldehyde or ketone is entitled the anomeric C atom.
a) True
b) False
View Answer
Explanation: Meanwhile an epimer is a stereoisomer that is very different in configuration at any single stereogenic center, an anomer is a cyclic saccharide and an epimer that is different in configuration, specifically at the hemiacetal/acetal carbon, also known the anomeric carbon.
3. The cyclic forms of carbohydrates are represented by Kraft projections.
a) True
b) False
View Answer
Explanation: A Haworth projection is a common way of formulating a structural formula to show the cyclic structure of monosaccharides with a simple 3-D perspective.The cyclic forms of carbohydrates are often represented by Haworth projections.
4. The acid- or base-catalyzed conversion of 1 anomer into its equilibrium mixture of anomers is known _________
a) Optical rotation
b) Muto rotation
c) Hetero rotation
d) Homo rotation
View Answer
Explanation: The acid- or base-catalyzed conversion of 1 anomer in its equilibrium mixture of anomers is known as mutarotation. It is accompanied by change in the optical rotation.
5. More complex carbohydrates occur in nature when 2 or more simple sugars are linked altogether. In nature, the linkages are _________ linkages, that’s, acetal or ketal bonds involving the anomeric carbon of just one of the monosaccharides involved. These bonds are called _________ linkages.
a) Glycosidic
b) Optical
c) Muto
d) Hydrosilic
View Answer
Explanation: A glycosidic linkage is a kind of a co-valent bond which joins a carbohydrate or sugar molecule to another group, which could or could not be another carbohydrate.
6. _________ linkages of compounds in aqueous solution are subject to hydrolysis in the presence of acid at flow back temp.
a) Glycosidic
b) Optical
c) Muto
d) Hydrosilic
View Answer
Explanation: Hydrolysis is the breaking of a bond by the addition of a H2O across it. Glycosidic linkages of compounds in aqueous solution are subject to hydrolysis in the presence of acid at flow back temperature.
7. What is the name of the compound?
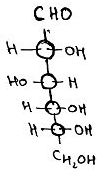
a) D-glucose
b) D-fructose
c) L-glucose
d) 4-O-methyl-D-glucuronic
View Answer
Explanation: The D-isomer (D-glucose), also called dextrose which occurs abundant in nature, but the L-isomer (L-glucose) doesn’t. Glucose’s formed during photosynthesis from H2O and CO2, utilizing energy from sunlight.
8. What is the name of the compound?
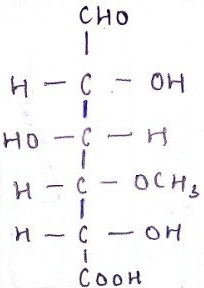
a) D-glucose
b) 1-O-methyl-L-glucuronic
c) L-glucose
d) 4-O-methyl-D-glucuronic
View Answer
Explanation:
Molecular Formula C7H12O7
Molecular Weight 208.17
C.A.S. Number 4120-73-4
Chemical Purity Min. 95% [1H-NMR] Appearance White Solid
9. What is the name of the compound?
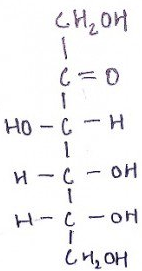
a) D-glucose
b) D-fructose
c) L-glucose
d) 4-O-methyl-D-glucuronic
View Answer
Explanation: Fructose’s a simple ketonic monosaccharide which is found in numerous plants, where it’s usually bonded to glucose. It’s one of the 3 dietary monosaccharides, along with glucose and galactose, that are directly absorbed into our bloodstream at the time of digestion. It could easily be found in honey, tree and vine fruits, flowers, berries, and most root vegetables.
10. What is the name of the compound?
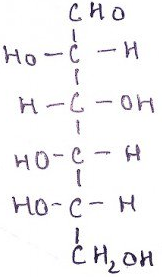
a) D-glucose
b) D-fructose
c) L-glucose
d) 4-O-methyl-D-glucuronic
View Answer
Explanation: L-Glucose is an organic compound with formula C6H12O6 or H–(C=O)–(CH-OH)5–H, specifically one of the aldohexose monosaccharides. As the L-isomer of glucose, it is an enantiomer of the more common D-glucose. L-Glucose is not present in higher order living organisms, but can be synthesized in the laboratory.
Sanfoundry Global Education & Learning Series – Pulp and Paper.
To practice all areas of Pulp and Paper, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
