This set of Enzyme Technology Interview Questions and Answers for freshers focuses on “Fundamentals – Enzyme Inhibition – 2”.
1. What happens to Vmax and Km in case of uncompetitive inhibition when I = Ki‘?
a) Lowers to 0.5 Vmax and 0.5 Km
b) Vmax is unchanged and Km increases 2Km
c) Lowers to 0.5 Vmax and Km remains unchanged
d) Lowers to 0.67 Vmax and Km increases to 2Km
View Answer
Explanation: Uncompetitive inhibition occurs during multi-substrate reaction where the inhibitor is competitive to one substrate and uncompetitive to the other. The inhibition cannot be overcome as Vmax and Km are equally reduced. The rate equation is given by, \(V=\frac{\left (\frac{V_{max}}{1+\frac{[I]}{K_i’}}\right ) [S]}{\left (\frac{K_m}{1+\frac{[I]}{K_i’}}\right )+[S]}\).
When I = Ki‘ in this equation, the Vmax and Km is reduced to half.
2. If I = Ki = Ki‘, then what will happen to Vmax and Km when inhibitor acts non-competitively?
a) Lowers to 0.5 Vmax and 0.5 Km
b) Vmax is unchanged and Km increases 2Km
c) Lowers to 0.5 Vmax and Km remains unchanged
d) Lowers to 0.67 Vmax and Km increases to 2Km
View Answer
Explanation: Non-competitive inhibitors are those inhibitors which bind at site away from the binding site, causing reduction in catalytic activity. It is a rare case mixed inhibition and the rate equation is given by, \(V=\frac{\left (\frac{V_{max}}{1+\frac{[I]}{K_i}}\right ) [S]}{K_m+[S]}\). When If I = Ki = Ki‘, Vmax is lowered to 0.5 Vmax and Km remains unchanged.
3. Mercury causes irreversible inhibition.
a) True
b) False
View Answer
Explanation: Irreversible inhibition is one in which loss of activity cannot be restored by the removal of inhibitor. Irreversible inhibition behaves as time-dependent loss of enzyme concentration, when the enzyme is totally inactive. In cases involving incomplete activation, there may be time dependent changes in both Km and Vmax. Heavy metal ions such as mercury, lead, etc., cause irreversible inhibition because they strongly bind to the amino acid backbone. Hence they should not come in contact with enzymes.
4. The following rate equation is given by ___________ inhibition.
\(V=\frac{\left (\frac{V_{max}}{1+\frac{[I]}{K_i’}}\right ) [S]}{\left (\frac{K_m}{1+\frac{[I]}{K_i’}}\right )+[S]}\)
a) mixed
b) competitive
c) uncompetitive
d) non-competitive
View Answer
Explanation: Uncompetitive inhibition is one wherein the inhibitor is competitive to one substrate but uncompetitive to other. In this case, Ki is much greater than the total inhibitor concentration and EI complex is not formed. This kind of inhibition is noticeable high substrate concentration and cannot be recovered as both Vmax and Km are equally reduced. Hence the rate equation becomes
\(V=\frac{\left (\frac{V_{max}}{1+\frac{[I]}{K_i’}}\right ) [S]}{\left (\frac{K_m}{1+\frac{[I]}{K_i’}}\right )+[S]}\)
Where,
Km = Miachelis Menton constant, Ki‘= Dissociation constant for ESI complex, I = Inhibitor concentration and S = Substrate concentration.
5. The rate equation for _______________ inhibition is given by \(V=\frac{V_{max} [S]}{K_m (1+\frac{[P]}{K_p})+[S]}\).
a) substrate
b) non-competitive
c) product
d) competitive
View Answer
Explanation: Product inhibition is case of competitive inhibition, where the substrate and the inhibitor compete for the active site of the enzyme. The inhibitors in this case bear structural similarity to the substrate. This causes substantial loss of productivity and hence the rate equation can be given by \(V=\frac{V_{max} [S]}{K_m (1+\frac{[P]}{K_p})+[S]}\)
Where,
Km = Miachelis Menton constant, Kp = Dissociation constant for product, P = Product concentration and S = Substrate concentration.
6. In the following diagram, which inhibition is represented?
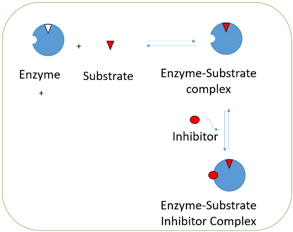
a) Mixed inhibition
b) Uncompetitive inhibition
c) Non-competitive inhibition
d) Product inhibition
View Answer
Explanation: In the above diagram, it is shown that inhibitor is bound to enzyme substrate complex rather than free enzyme. This occurs when one substrate in bound to the active site which makes the inhibitor to bind to site of the enzyme. It usually occurs during multi-substrate reaction. Hence the diagram shows uncompetitive inhibition. Mixed inhibition is one where in both EI and ESI complexes are formed. Non-competitive is a special case of mixed inhibition. Product inhibition is case of competitive inhibition where in the substrate and the enzyme compete for the active site of the enzyme.
7. Inhibition of lactase by galactose is an example of which kind of inhibition?
a) Uncompetitive inhibition
b) Mixed inhibition
c) Competitive inhibition
d) Substrate inhibition
View Answer
Explanation: In competitive inhibition, the inhibitor is having structural similarity to substrate, and often is a reaction product. As lactose and galactose have structural similarity, they exhibit competitive inhibition. Substrate inhibition is a case uncompetitive inhibition. Uncompetitive inhibition occurs wherein one substrate is competitive and other substrate is non-competitive with respect to the enzyme. Mixed inhibition is said to occur when both enzyme-inhibitor and enzyme-substrate-inhibitor complexes are formed.
8. The following diagram represents _______________ inhibition.
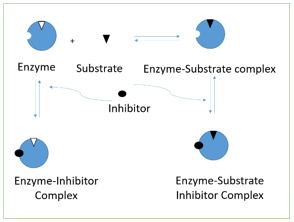
a) competitive
b) non-competitive
c) product
d) uncompetitive
View Answer
Explanation: Non-competitive inhibition is represented in the diagram. As the diagram suggest, EI and ESI complexes are formed. This kind of inhibition occurs when the inhibitor is bound to the enzyme at site, far away from it catalytic activity. It is a special case of mixed inhibition. In competitive inhibition, only EI complex is formed as compared to Uncompetitive where only ESI complex is formed. Product inhibition is case of competitive inhibition.
9. Loss of activity which may be restored by the removal of inhibitor is referred to as ___________
a) reversible inhibition
b) irreversible inhibition
c) competitive inhibition
d) mixed inhibition
View Answer
Explanation: Reversible inhibition is one where in loss of activity of activity may be revived by removal of inhibitor. As compared to, irreversible inhibition where the loss of activity cannot be restored. Mixed inhibition is one where in both EI and ESI complexes are formed with no product formation. Competitive inhibition occurs when both substrate and the inhibitor compete for binding to active site.
Sanfoundry Global Education & Learning Series – Enzyme Technology.
To practice all areas of Enzyme Technology for Interviews, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Enzyme Technology Books
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Practice Biotechnology MCQs
- Apply for Chemical Engineering Internship
