This set of Enzyme Technology Multiple Choice Questions & Answers (MCQs) focuses on “Fundamentals – Enzyme Inhibition – 1”.
1. Substances which reduce the rate of enzyme catalyzed reactions are known as ____________
a) substrates
b) enzymes
c) products
d) inhibitors
View Answer
Explanation: Certain substances may reduce the rate of enzyme catalyzed reactions. Some of these are non-specific protein denaturants (e.g., urea). While other substances which act in a fairly specific manner are referred to as inhibitors. Enzymes are biocatalyst which act on substrates to form products.
2. In the following diagram, which kind of inhibition is represented?
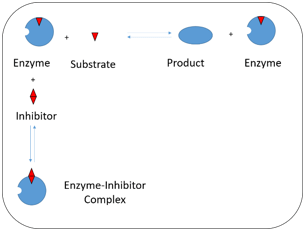
a) Uncompetitive inhibition
b) Competitive inhibition
c) Non-competitive inhibition
d) Mixed inhibition
View Answer
Explanation: In the above diagram, it is shown that both substrate and inhibitor compete for the active site of the enzyme with formation of enzyme-inhibitor [EI] complex rather than enzyme-substrate-inhibitor [ESI] complex. Hence the inhibition shown in the diagram is competitive inhibition. It usually occurs at low substrate concentrations, but can be overcome at high substrate concentration.
3. The following rate equation represents _____________ inhibition.
\(V=\frac{V_{max} [S]}{K_m (1+\frac{I}{K_i})+[S]}\)
a) competitive
b) mixed
c) non-competitive
d) uncompetitive
View Answer
Explanation: Competitive inhibition occurs when both substrate and inhibitor compete for binding to the active site of the enzyme. In this kind of inhibition, Ki is much greater than total inhibitor concentration and ESI complex is not formed. This kind of inhibition is mostly seen when the substrate concentration are low. This inhibition can be overcome at sufficiently high substrate concentrations as Vmax remain unaffected. Hence the rate equation is represented by \(V=\frac{V_{max} [S]}{K_m (1+\frac{I}{K_i})+[S]}\) Where,
Km = Miachelis Menton constant, Ki = inhibitor constant, I = Inhibitor concentration and S = Substrate concentration.
4. Uncompetitive inhibition is most noticeable at low substrate concentration and can be overcome at high substrate concentration.
a) True
b) False
View Answer
Explanation: Uncompetitive inhibitions occurs during multi-substrate reactions where the inhibitor is competitive to one substrate and uncompetitive to the other. These inhibition occur when one substrate is already bound to the enzyme. Ki is much greater than the total inhibitor concentration and EI complexes are not formed. Hence the above statement is false. This is true in case of competitive inhibition.
5. Inhibition of invertase by sucrose falls into which category of inhibition?
a) Substrate inhibition
b) Non-competitive inhibition
c) Product inhibition
d) Competitive inhibition
View Answer
Explanation: Invertase inhibition by sucrose is kind of substrate inhibition. It is a special case of uncompetitive inhibition which occurs at high substrate concentrations. Product inhibition is case of competitive inhibition wherein the substrate and the inhibitor have structural similarity. Competitive inhibition is one wherein the substrate and the inhibitor compete for the active site of the enzyme. Non-competitive inhibition occurs when the inhibitor binds at the site away from the binding site, causing a reduction in catalytic activity.
6. ______________ inhibition represent the following rate equation.
\(V=\frac{\left (\frac{V_{max}}{1+\frac{[I]}{K_i}}\right )[S]}{K_m+[S]}\)
a) Substrate inhibition
b) Competitive inhibition
c) Product inhibition
d) Non-competitive inhibition
View Answer
Explanation: Non-competitive inhibition is a special case of mixed inhibition. This kind of inhibition occurs when the inhibitor binds to the enzyme, far away from the catalytic site. In this case both EI and ESI complexes are equally formed. Hence the rate equation is given by the equation
\(V=\frac{\left (\frac{V_{max}}{1+\frac{[I]}{K_i}}\right )[S]}{K_m+[S]}\)
Where,
Km = Miachelis Menton constant, Ki = inhibitor constant, I = Inhibitor concentration and S = Substrate concentration.
7. The __________ inhibition gives the following rate equation.
\(V=\frac{V_{max} [S]}{K_m+[S](1+\frac{[S]}{K_s})}\)
a) Substrate
b) Mixed
c) Non-competitive
d) Competitive
View Answer
Explanation: Substrate inhibition is a special case of uncompetitive inhibition which occurs in about 20% of all known enzymes. It is primarily caused by binding of parts of the substrate molecule to the subsites in the active site. At high substrate concentration, the resultant complex may become inactive causing reduction in the rate of reaction. Assuming the ESS complex may not form product, the rate equation may be given by \(V=\frac{V_{max} [S]}{K_m+[S](1+\frac{[S]}{K_s})}\)
Where,
Km = Miachelis Menton constant, Ks = Dissociation constant for substrate and S = Substrate concentration.
8. In competitive inhibition, what happens to Vmax and Km if [I] = Ki?
a) Lowers to 0.5 Vmax and 0.5 Km
b) Vmax is unchanged and Km increases 2Km
c) Lowers to 0.5 Vmax and Km remains unchanged
d) Lowers to 0.67 Vmax and Km increases to 2Km
View Answer
Explanation: Competitive inhibition is one wherein the inhibitor and the substrate compete for the active site. Inhibitor and substrate are said to be structurally similar. Thus, the rate equation for competitive inhibition is given by \(V=\frac{V_{max} [S]}{K_m (1+\frac{I}{K_i})+[S]}\). According to this equation, Vmax remains unchanged and Km increases 2Km.
9. Which of these is correct for mixed inhibition?
a) Lowers to 0.5 Vmax and 0.5 Km
b) Vmax is unchanged and Km increases 2Km
c) Lowers to 0.5 Vmax and Km remains unchanged
d) Lowers to 0.67 Vmax and Km increases to 2Km
View Answer
Explanation: Mixed inhibition is said to occur when both EI and ESI complexes are formed. The rate equation is this case is given by \(V=\frac{V_{max} [S]}{K_m (1+\frac{[I]}{K_i})+[S](1+\frac{[I]}{K_i’})}\)
Where
Km = Miachelis Menton constant, Ki = Dissociation constant for EI complex, [I] = Inhibitor concentration, S = Substrate concentration and Ki‘ = Dissociation constant for ESI complex
Now, if Ki = [I] = 0.5 Ki‘, then Vmax lowers to 0.67 and Km increases to 2Km.
Sanfoundry Global Education & Learning Series – Enzyme Technology.
To practice all areas of Enzyme Technology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Enzyme Technology Books
- Practice Biotechnology MCQs
- Apply for Chemical Engineering Internship
- Apply for Biotechnology Internship
- Check Biotechnology Books
