This set of Enzyme Technology Multiple Choice Questions & Answers (MCQs) focuses on “Recent Advances – Equilibria in Biphasic Aqueous-Organic Systems”.
1. In the below reaction, Kw is represented by ___________
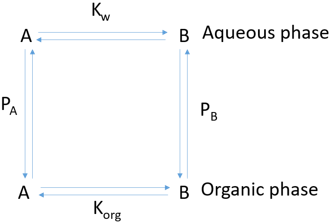
a) \(\frac{[B_w]}{[A_w]}\)
b) \(\frac{[B_{org}]}{[A_{org}]}\)
c) \(\frac{[A_{org}]}{[A_w]}\)
d) \(\frac{[B_{org}]}{[B_w]}\)
View Answer
Explanation: The reaction below represents a biphasic system consisting of a mixture of an aqueous and an organic solvent.

Kw and Korg represents equilibrium constants for water and organic phase respectively. PA and PB are partition co-efficients of A and B respectively.
Korg = \(\frac{[B_{org}]}{[A_{org}]}\)
PA = \(\frac{[A_{org}]}{[A_w]}\)
PB = \(\frac{[B_{org}]}{[B_w]}\)
Equilibrium constant for water Kw is given by \(\frac{[B_w]}{[A_w]}\),
Where Aw and Bw are concentrations of A and B.
2. What does PA represent in the following reaction?

a) \(\frac{[B_w]}{[A_w]}\)
b) \(\frac{[B_{org}]}{[A_{org}]}\)
c) \(\frac{[A_{org}]}{[A_w]}\)
d) \(\frac{[B_{org}]}{[B_w]}\)
View Answer
Explanation: The above reaction represents a biphasic system consisting of a mixture of an aqueous and an organic solvent. Kw and Korg represents equilibrium constants for water and organic phase respectively. PA and PB are partition co-efficients of A and B respectively.
Korg = \(\frac{[B_{org}]}{[A_{org}]}\)
PB = \(\frac{[B_{org}]}{[B_w]}\)
Kw = \(\frac{[B_w]}{[A_w]}\)
Partition coefficient for A is represented by PA = \(\frac{[A_{org}]}{[A_w]}\),
Where Aorg and Aw are the concentration of A in organic solvent and water respectively.
3. The apparent equilibrium constant (Kbiphasic) of this system may be defined as _____________
a) \(\frac{[B_{org}]}{[A_{org}]}\)
b) \(\frac{[B_w]}{[A_w]}\)
c) \(\frac{[B_t]}{[A_t]}\)
d) \(\frac{[B_{org}]}{[B_w]}\)
View Answer
Explanation: The biphasic system is represented by the following reaction

Kw and Korg represents equilibrium constants for water and organic phase respectively. PA and PB are partition co-efficients of A and B respectively.
Kbiphasic represents the apparent equilibrium constant and is given by, \(\frac{[B_t]}{[A_t]}\),
Where At = Concentration of A in total solution and Bt = Concentration of B in total solution.
4. Which of the following does not represent Kbiphasic?
a) \(\frac{[B_{org}]}{[A_{org}]}\)
b) \(\frac{[B_t]}{[A_t]}\)
c) \(K_w (\frac{1+αP_B}{1+αP_A})\)
d) \(K_w \frac{P_B}{P_A}\)
View Answer
Explanation: Kbiphasic is the apparent equilibrium constant and is represented in the following ways:
\(K_{biphasic} = \frac{[B_t]}{[A_t]}\)
\(K_{biphasic} = K_w (\frac{1+αP_B}{1+αP_A})\)
\(K_{biphasic} = K_w \frac{P_B}{P_A}\)
Equilibrium constant for organic solvent reaction is given by Korg = \(\frac{[B_{org}]}{[A_{org}]}\),
Where Aorg and Borg gives the concentration of A and B in organic solvents respectively.
5. What does α represent in the below equation?
\(K_{biphasic} = K_w (\frac{1+αP_B}{1+αP_A})\)
a) \(\frac{[B_w]}{[A_w]}\)
b) \(\frac{V_{org}}{V_w}\)
c) \(\frac{[B_t]}{[A_t]}\)
d) \(\frac{[B_{org}]}{[B_w]}\)
View Answer
Explanation: Kbiphasic in terms of volumes is represented by
\(K_{biphasic} = K_w (\frac{1+αP_B}{1+αP_A})\),
Where Kw = equilibrium constant of water, PA and PB are partition coefficients of A and B respectively.
α is the ratio of the volumes of the organic (Vorg) and water (Vw) phases and is given by \(\frac{V_{org}}{V_w}\).
Kw = \(\frac{[B_w]}{[A_w]}\) ; \(K_{biphasic}=\frac{[B_t]}{[A_t]}\) ; and PB = \(\frac{[B_{org}]}{[B_w]}\).
6. What does the following graph represents?
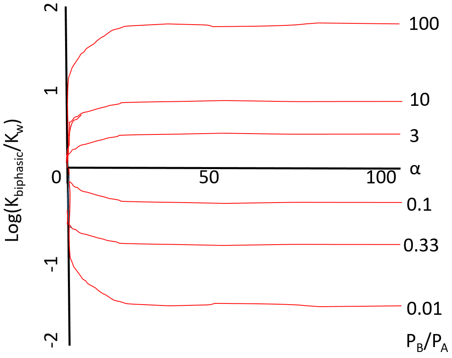
a) Effect of the polarity of A and B
b) Variation in the apparent equilibrium constants
c) Effect of the ratio of the partition coefficients
d) Variation in the apparent equilibrium constant and percentage yield
View Answer
Explanation: The graph represents the effect of the ratio of the partition coefficients (PB/PA). The curves are generated by using the equation \(K_{biphasic} = K_w (\frac{1+αP_B}{1+αP_A})\). In the plot, the variation in the apparent equilibrium constants of a one-substrate one-product reaction with the relative component composition of a biphasic aqueous-organic system is shown.
7. What is represented in the following plot?
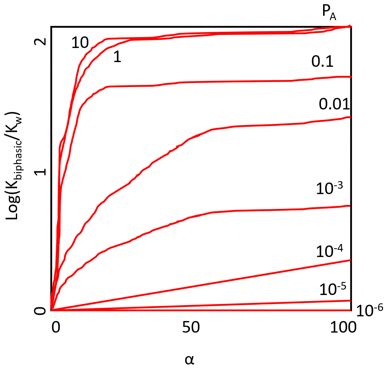
a) Effect of the polarity of A and B
b) Variation in the apparent equilibrium constants
c) Effect of the ratio of the partition coefficients
d) pH dependence on the apparent equilibrium constant
View Answer
Explanation: The plot represents the effect of polarity he polarity of A and B, keeping the ratio of the partition coefficients constant (PB/PA = 100). This plot is drawn considering the variation in the apparent equilibrium constants of a one-substrate one-product reaction with the relative component composition of a biphasic aqueous-organic system. The following values for the partition coefficients have been used, from the top downwards; PB = 100, PB = 10, PB = 1, PB = 0.1, PB = 0.01, PB = 0.001, PB = 0.0001.
8. What does the diagram depict?
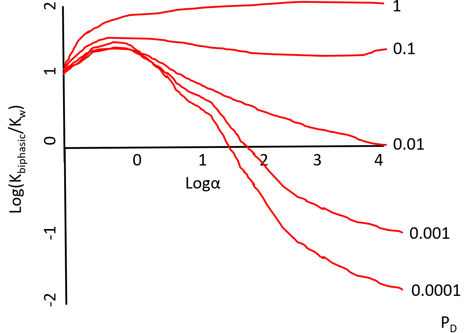
a) Variation in the apparent equilibrium constants of a one-substrate one-product reaction
b) Variation in the apparent equilibrium constants of a two-substrate two-product reaction
c) pH dependence on apparent equilibrium constant
d) Effect of the polarity of A and B
View Answer
Explanation: The diagram depicts a plot which shows the variation in the apparent equilibrium constants of a two-substrate two-product reaction with the relative component composition of a biphasic aqueous-organic system. The 2 substrate 2 product reaction may be given as,
A+B ⇋C+D
In all cases the partition coefficients are PA = 1, PB = 1 and PC = 100. The following values for the partition coefficient have been used, from the top downwards; 1, 0.1, 0.01, 0.001 and 0.0001.
9. What is represented in the plot?
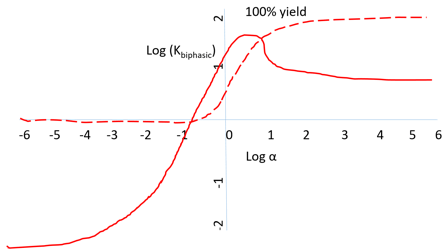
a) Variation in the apparent equilibrium constants of a one-substrate one-product reaction
b) Variation in the apparent equilibrium constants of a two-substrate two-product reaction
c) Variation in the apparent equilibrium constant and percentage yield
d) pH dependence on apparent equilibrium constant
View Answer
Explanation: In the plot, variation in the apparent equilibrium constant and percentage yield is represented which is semi-logarithmic plot. This plot is portrayed for a reaction of synthesizing N-Benzoyl-L-phenylalanine ethyl ester by the action of chymotrypsin on N-Benzoyl-L-phenylalanine and ethyl alcohol in a biphasic chloroform-water system (pH 7). Straight red line represents apparent equilibrium constant, whereas red dotted line represents % yield.
10. What does the plot in the diagram depict?
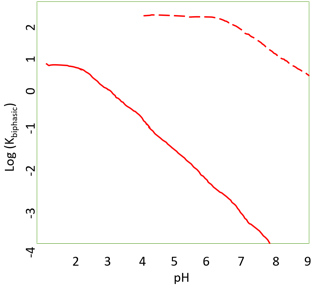
a) Variation in the apparent equilibrium constants of a one-substrate one-product reaction
b) Variation in the apparent equilibrium constants of a two-substrate two-product reaction
c) Variation in the apparent equilibrium constant and percentage yield
d) pH dependence on apparent equilibrium constant
View Answer
Explanation: The plot in the diagram depicts pH dependence on apparent equilibrium constant or the synthesis of N-Benzoyl-L-phenylalanine ethyl ester, as given by the reaction scheme. Red straight line represents reaction in aqueous solution only, whereas red dotted line reaction in a biphasic chloroform-water system. An upward shift is seen which is because of the more significant partitioning of the ester into the organic phase relative to the reactants. The shift in the pH dependence is higher pH is due to the change in the apparent pKa of the acid.
Sanfoundry Global Education & Learning Series – Enzyme Technology.
To practice all areas of Enzyme Technology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Biotechnology MCQs
- Check Biotechnology Books
- Practice Chemical Engineering MCQs
