This set of Enzyme Technology Multiple Choice Questions & Answers (MCQs) focuses on “Mechanism of Enzyme Catalysis”.
1. ___________ catalyze the reaction by accepting a proton.
a) Acid
b) Entrapment
c) Enzyme
d) Bases
View Answer
Explanation: Bases catalyze the reaction by accepting a proton, whereas acid by donating a proton. Enzymes are biological catalyst which increases the rate of the reaction, without undergoing any change in itself. Entrapment is an immobilization method that entraps enzyme in a confined space.
2. Which of the following is not true for acid base catalysis?
a) Bases catalyze the reaction by accepting a proton
b) Bases increases the reaction rate by increasing the nucleophilic character of the attacking group
c) To make reactants proximal to each other
d) Specific hydroxide ion catalysis of reaction in water is an example of this type of catalysis
View Answer
Explanation: “To make reactants proximal to each other.” This is true for catalysis by approximation, and not acid base catalysis. Following statements are true for acid base catalysis:
• Bases catalyze the reaction by accepting a proton, whereas acids by donating a proton.
• Bases increases the reaction rate by increasing the nucleophilic character of the attacking group.
• Specific hydroxide ion catalysis of reaction in water is an example of this type of catalysis. The rate law will have two terms: unanalyzed rate and hydroxide catalyzed term.
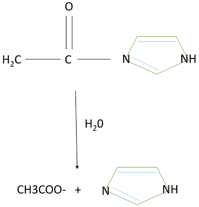
3. The reaction in the diagram belongs to which type of catalysis?
a) Strain distortion and conformational change
b) Approximation catalysis
c) Acid base catalysis
d) Covalent catalysis
View Answer
Explanation: The reaction represents the case of hydrolysis of acetyl imidazole which explains the acid base catalysis. The imidazole functions as a base catalyst in addition to OH. Catalysis by imidazole implies that the energy barrier for hydrolysis is lowered by proton itself. Therefore, the nuclepohilicity of the water molecule is enhanced without the generation of a high concentration of OH. The catalytic transition state is stabilized by avoiding the formation of unstable high energy species due to the hydrogen bond in the transition state.
4. Which of the following is not true for strain distortion and conformational change?
a) Bases increases the reaction rate by increasing the nucleophilic character of the attacking group
b) The strain in the starting material and release of that strain provides rate acceleration in chemical reactions
c) This experiment was carried out by Westheimer and his colleagues
d) Rate of hydrolysis of phosphate esters in two compounds was explained by this theory
View Answer
Explanation: “Bases increases the reaction rate by increasing the nucleophilic character of the attacking group.” This is true for acid base catalysis. The statements which are true for strain distortion and conformational change are as follows:
• The strain in the starting material and release of that strain provides rate acceleration in chemical reactions.
• This experiment was carried out by Westheimer and his colleagues.
• Rate of hydrolysis of phosphate esters in two compounds was explained by this theory. The ring opening of cyclic example on hydrolysis has relative rate of 108 fold faster than acyclic example.
5. Which of the following is not true for strain distortion and conformational change?
a) Change in conformation 3D structure of a protein from low activity form to high activity form
b) To make reactants proximal to each other
c) Intrinsic interaction energy and geometric distortions are involved
d) ES complex is selectively destabilized
View Answer
Explanation: “To make reactants proximal to each other.” This is not true for strain distortion and conformational change. The statements which are true are as follows:
• Change in conformation 3D structure of protein from low activity form to high activity form. Hence strain and distortion effects are important in enzymatic catalysis.
• Intrinsic interaction energy is used to accelerate catalysis. Whereas, geometric distortions of bond angles in bound substrate or steric compression has some effect on destabilization of ES complex.
• ES complex is selectively destabilized, if the destabilization forces are released in transition state.
6. Which of the following statement is not true for catalysis by approximation?
a) Change in conformation 3D structure of protein from low activity form to high activity form
b) To make reactants proximal to each other
c) This proximity raises effective concentration over that of the reactants free in solution and lead to rate acceleration
d) To quantify some proximity effects, model studies were carried out
View Answer
Explanation: “Change in conformation 3D structure of a protein from low activity form to high activity form.” This is true for strain distortion and conformational change. Catalysis by approximation has the following features:
• To make reactants proximal to each other as they will be at the active site.
• This proximity raises effective concentration over that of the reactants free in solution and lead to rate acceleration.
• To quantify some proximity effects, model studies were carried out.
7. Which of the following is not involved in covalent catalysis?
a) Bases which catalyze the reaction by accepting a proton
b) Electron rich nucleophilic function group of amino acid side chain
c) Electron deficient electrophilic portion of substrate
d) Acylated, phosphorylated or glycosylated enzyme nucleophile as covalent intermediate
View Answer
Explanation: The following are involved in covalent catalysis:
• Electron rich nucleophilic function group of amino acid side chain.
• Electron deficient electrophilic portion of substrate.
• Acylated, phosphorylated or glycosylated enzyme nucleophile as covalent intermediate.
“Bases which catalyze the reaction by accepting a proton.” These are involved in acid base catalysis.
8. Which of the following is not true for covalent catalysis?
a) A number of coenzymes for covalent adducts generating new electrophilic groups which can function as electron sink
b) These adduct forming coenzymes leads to increase in rate acceleration
c) About 100 enzymes show covalent intermediates during catalysis
d) Hydrolysis of acetyl imidazole is an example of this type of catalysis
View Answer
Explanation: Hydrolysis of acetyl imidazole is an example of acid base catalysis, and not covalent catalysis. The following statements are true for covalent catalysis:
• A number of coenzymes for covalent adducts generating new electrophilic groups which can function as electron sink.
• These adduct forming coenzymes leads to increase in rate acceleration by route low energy, stabilized, substrate derived carbonions.
• About 100 enzymes show covalent intermediates during catalysis.
Sanfoundry Global Education & Learning Series – Enzyme Technology.
To practice all areas of Enzyme Technology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Biotechnology Internship
- Check Biotechnology Books
- Apply for Chemical Engineering Internship
- Check Enzyme Technology Books
- Practice Chemical Engineering MCQs
