This set of Enzyme Technology Question Bank focuses on “Kinetics of Immobilized Enzymes – 3”.
1. Immobilization affects the kinetic constants (Km and Vmax) of the enzyme.
a) True
b) False
View Answer
Explanation: The above statement is true. For example: Trypsin in free form hydrolyses 15 peptides of pepsinogen whereas trypsin in immobilized form hydrolyses 10 bonds. This is due to change in properties of solution in the immediate vicinity of immobilized enzymes or due to molecular diffusion within local environment.
2. __________ is the average ratio of path length between any points within the particle to their absolute distance apart.
a) Porosity
b) Kcat
c) Tortuosity
d) Catalytic efficiency
View Answer
Explanation: Tortuosity is the average ratio of path length between any points within the particle to their absolute distance apart. The tortuosity is always greater than or equal to unity which is clearly shows that it depends on pore geometry. Porosity is the ratio of volume of solution contained within the particle to the total volume of the particle. Kcat is referred to as turn over number that denotes the number of catalytic turn over events that happens per unit time. Kcat/Km is termed as catalytic efficiency.
3. What does the following equation represent?
V=\(\frac{V_{max}}{1+\frac{K_m^{app}}{[S_0]}}\)
a) Lineweaver Burk equation
b) Electrostatic potential coefficient
c) Miachelis Menten equation for enzymes
d) Miachelis Menten equation for immobilized enzymes
View Answer
Explanation: Miachelis Menten equation for enzymes is given by V=\(\frac{V_{max} S}{K_m+S}\)
For immobilized enzymes, the substrate concentration is the concentration within the microenvironment(S0) and the equation is given by, V=\(\frac{V_{max}}{1+\frac{K_m^{app}}{[S_0]}}\) Where, \(K_m^{app}\) = apparent Km, Vmax = maximum rate of reaction, V = rate of a reaction and Km = Miachelis Menten constant. Electrostatic potential coefficient is given by Ʌ =\(\frac{[C_0^{n+}]}{[C^{n+}]}= \frac{[A^{n-}]}{[A_0^{n-}]}\) where,
\(C_0^{n+}\) and \(A_0^{n-}\) are concentration of anions and cations in bulk environment
Cn+ and An- are concentration of anions and cations in microenvironment
Lineweaver Burk equation is derived by reciprocating the Miachelis Menten equation.
4. What does the following diagram represent?
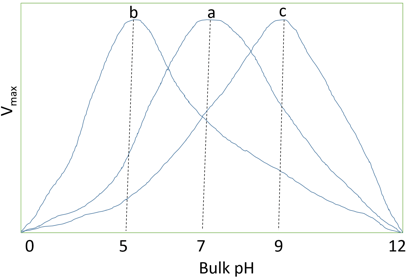
a) Activity profile of immobilized enzymes against pH
b) Lineweaver Burk plot
c) Effect of multipoint interaction for stabilization of enzymes
d) Effect of temperature on activity of enzymes
View Answer
Explanation: The pH of micro and bulk environment depends on whether H+ ions are being attracted or repelled by the charge on immobilized enzyme. Now, as the binding substrate and activity of immobilized enzyme depends on the local micro-environmental pH, this portioning cause an appropriate shift in Km with respect to other solution. This is a commonly used method to alter the pH. The diagram represents the profile of activity of an enzyme, immobilized on charged supports with pH of the solution. In this diagram, ‘a’ represents free enzyme, ‘b’ represents enzyme bound to anionic support and ‘c’ represents enzyme bound to cationic support. In case ‘b’, a bulk of pH 5 is needed to produce a product of pH 7 in microenvironment. In case ‘c’, a bulk pH of 9 is needed to produce a pH of 7 in microenvironment.
5. What does the equation, \(D_a = \frac{V_{max}}{K_L [S_b]}\) represent?
a) Porosity
b) Turnover number
c) Damkohler number
d) Tortuosity
View Answer
Explanation: Damkohler number depends on the rate of the reaction and diffusion rate and is given by,
Da=(maximum rate of reaction)/(maximum rate of diffusion)=\(\frac{V_{max}}{K_L [S_b]}\), where
Vmax = Maximum rate of reaction
KL = Mass transfer coefficient (cm/s)
Sb = Substrate concentration in bulk liquid (g/cm3)
Damkohler number has a significant effect on diffusional resistance. If the Da >> 1, the diffusion rate is limiting. Whereas if the Da ≪ 1, the reaction rate is limiting. For a = 1, the diffusion and the reaction resistances are comparable.
6. What does ‘X’ represent in the following graph?
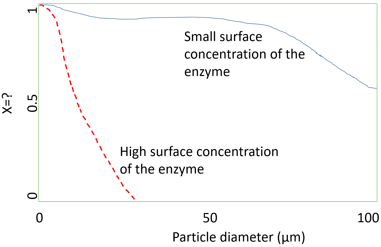
a) Turnover number
b) Damkohler number
c) Vmax
d) Effectiveness factor
View Answer
Explanation: The above graph shows the effect of particle diameter on the effectiveness factor of immobilized enzymes. Effectiveness factor is given by \(ƞ=\frac{V}{V_{free}}\), where
V = rate of reaction catalyzed by immobilized enzymes
Vfree = rate of reaction catalyzed by free enzymes
The red dotted line and blue non-dotted line represents the effect of particle diameter on the effectiveness factor at high surface concentration of the enzyme and at small surface concentration of the enzyme. According to the graph, as particle diameter decreases the effectiveness factor also decreases for high surface concentration and for small surface concentration, initially it is maintained and then decreases gradually. The ‘X’ in the plot represents effectiveness factor.
7. In case of immobilized enzymes, the equation \(V=K_L ([S_0]-[S])=\frac{V_{max} [S]}{K_m+S+\frac{S^2}{K_S}}\) represents ______________ inhibition.
a) substrate
b) product
c) uncompetitive
d) competitive
View Answer
Explanation: As compared to product inhibition, the equation is complicated for substrate inhibition and is given by \(V=K_L ([S_0 ]-[S])=\frac{V_{max} [S]}{K_m+S+\frac{S^2}{K_S}}\) where,
Vmax = max rate of reaction, S = Substrate concertation, S0 = initial substrate concentration, Km = Miachelis Menten constant, KL = Substrate mass transfer co-efficient, and Kp = Product inhibitory constant.
This equation is third order with respect to the micro-environmental substrate concentration.
8. What does the following graph represent?
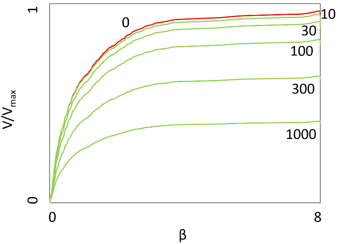
a) Concentration profile
b) Variation in rate of reaction with surface concentration of the substrate
c) Arrhenius plot
d) Lineweaver Burk plot
View Answer
Explanation: The plot in the above diagram represents the variation in rate of the reaction catalyzed by porous particles containing immobilized enzyme with surface concentration of the substrate (β), which is dimensionless. The top curve shows that there is no diffusional control (substrate modulus for internal diffusion = 0) and the following lower curves shows the effect progressively increasing substrate modulus equaling 10, 30, 100, 300, 1000 etc. The substrate modulus for internal diffusion is normalized with respect to Km in order to make independent of the absolute value of the exterior substrate concentration. Concentration profile comes into picture when the enzymes are immobilized on spherical surfaces. Arrhenius plots are related temperature and rate of reaction. Lineweaver Burk plot is a plot of deals with reversal of Miachelis Menten equation for enzyme kinetics.
9. The graph in the following diagram represents ____________________
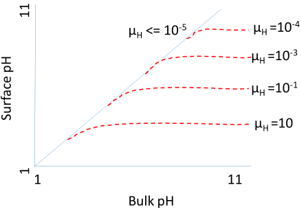
a) The effect of diffusional control on the local pH
b) The effect of diffusional control on pH activity profile of an immobilized enzyme
c) The effect of pH on activity of a free enzyme
d) Arrhenius plot
View Answer
Explanation: The graph in the diagram represents the effect of diffusional control on the local pH. This graph shows the variation of surface pH and bulk pH at various proton moduli (µH). The blue line represents the variation at µH = 10-5 and red dotted line represents the variation at different proton moduli (µH = 10, µH = 10-1v, µH = 10-3, µH = 10-4). At high pH, the micro-environmental pH reduced due to presence of very low hydrogen ion concentration gradient and hydrogen ion diffusion rates. From the plot, it is found that hydrogen ions accumulate at the surface when the proton modulus is greater than about 10,000. Hence the reactions must be operated at a significant rate away from the pH optimum of the free enzyme.
10. Within the micro-environment, the same bulk concentration may give two different stable concentrations.
a) True
b) False
View Answer
Explanation: Within the micro-environment, the same bulk concentration may give two different stable concentrations which are follows:
A low concentration which gives faster rate of reaction, with no substrate inhibition and equally fast rate of inward diffusion sue to steep concentration gradient.
A high concentration which gives slower rate of reaction due to substrate inhibition and equally slow rate of inward diffusion due to concentration gradient going downward.
There is a possibility of an existence of a third intermediate concentration depicting an unstable state which is not naturally established and is of no practical consequence.
Sanfoundry Global Education & Learning Series – Enzyme Technology.
To practice Enzyme Technology Question Bank, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Check Enzyme Technology Books
- Check Biotechnology Books
- Apply for Biotechnology Internship
