This set of Enzyme Technology Questions and Answers for Freshers focuses on “Enzyme Kinetics Fundamentals – Effect of pH, Temperature, Pressure, and Ionic Strength on Enzyme Catalysis”.
1. Enzymes are amphoteric molecules.
a) True
b) False
View Answer
Explanation: Enzymes are biological catalysts. Enzymes contain large number acidic and basic groups situated on their surface. They can either act as acid or base depending on the conditions. Hence enzymes are amphoteric in nature.
2. The pH at which the net charge on the enzyme molecule is zero is called __________
a) pKa
b) Half-life
c) Isoelectric point
d) Km
View Answer
Explanation: Enzymes are amphoteric in nature. As the pH conditions vary, they may behave as acids or bases. But, there is particular pH of an enzyme at which the net charge on the molecule becomes zero. This point is called as isoelectric point. At this point, enzymes will have minimal solubility in aqueous solutions. pKa is defined as the pH at which half of the groups are ionized. Km is the concentration of the substrate at which half of the enzyme are in bound state. The time taken to reduce the activity to half of its original activity is called half-life.
3. Calculate the ionic strength of 0.2 M solution of Calcium chloride?
a) 0.9 M
b) 0.06 M
c) 0.6 M
d) 6.0 M
View Answer
Explanation: Ionic strength is given by the equation I = 0.5∑ci zi2
Where ci = concentration of every ionic species, zi = Charge of every ionic species.
Charge of chlorine and calcium is -1 and +2 respectively.
Concentration of calcium = 0.2 * 1 = 0.2
Concentration of chlorine = 0.2 * 2 = 4
I = 0.5 (0.2[2]2 + 0.4 [-1]2)
I = 0.5(0.8 + 0.4)
I = 0.6 M.
4. The rate of enzyme catalyzed reaction decreases with an increase in ionic strength if the charges are identical.
a) True
b) False
View Answer
Explanation: The ionic strength depends on the binding of charged substrates to enzyme and movement of charged groups within the catalytic site. If the rate of the reaction depends on charged moieties, the rate of the reaction may be given as, log(k)=log(k0)+ZA ZB \(\sqrt{I}\)
Where, k = rate constant, k0 = rate constant at zero ionic strength, ZA and ZB = electrostatic charges on reacting species, I = ionic strength of the solution.
If the charges are opposite, there is an increase in the rate of the reaction with increasing ionic strength, while if the charges are identical, there is a decrease in the rate of the reaction with increasing ionic strength.
5. What does the following graph represent?
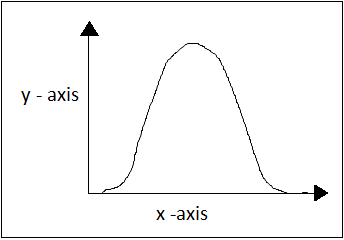
a) Effect of temperature on the rate of enzyme catalyzed reaction
b) Effect of pH on the rate of enzyme catalyzed reaction
c) Effect on ionic strength on the rate of enzyme catalyzed reaction
d) Effect of temperature on stability of an enzyme catalyzed reaction
View Answer
Explanation: The graph shows a bell-shaped curve which is exhibited by pH versus rate of enzyme catalyzed reaction (activity). As pH increases, enzyme activity increases and reaches a maximum point (optimum pH) and then starts reducing on further treatment with pH. The activity is reduced when pH > 8, because of destruction of cysteine residues due to base catalyzed β- elimination reaction. The activity also reduces when pH < 4, because peptide bonds next to aspartic acid are liable to hydrolysis.
6. The following graph represents the effect of ____________ on activity of an enzyme catalyzed reaction.
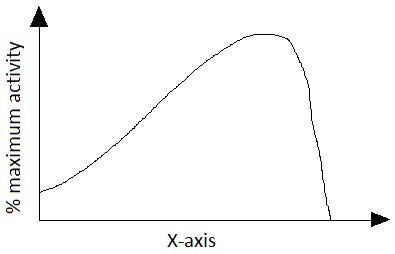
a) pH
b) Incubation period
c) Temperature
d) Productivity
View Answer
Explanation: The effect of temperature on activity of an enzyme catalyzed is given in the above graph. In an exothermic reaction, the reverse reaction increases more rapidly than forward reaction. Due to this equilibrium is altered and optimum temperature of the reaction is reduced. The reverse holds good for endothermic reaction. It is preferred to use enzymes at high temperatures. But, at temperatures above critical temperature, enzymes may undergo irreversible denaturation resulting in loss of activity. As the incubation time increases, the activity of an enzyme catalyzed reaction reduces at given temperatures. As the incubation time increases, the productivity initially increases and then reaches a stationary at given temperatures.
7. What does the equation, k = \(Ae^{\frac{-∆G}{RT}}\) represent?
a) Arrhenius equation
b) Miachelis Menton equation
c) Lineweaver Burk equation
d) Half-life
View Answer
Explanation: The Arrhenius equation is given by k = \(Ae^{\frac{-∆G}{RT}}\)
Where k = kinetic rate constant, A = Arrhenius constant, ΔG = standard free energy of activation (kJ M-1), R = universal gas constant and T = absolute temperature.
Rate of all the enzyme catalyzed reaction with an increase in temperature is given by the Arrhenius equation. Miachelis Menton equation and Lineweaver Burk equation represents enzyme activity whereas Half-life represents the time at enzyme activity is reduced to half.
8. For every, 1000 fold increase in pressure, the Km is _____________
a) reduced to half
b) doubled
c) reduced to zero
d) increased by 10 folds
View Answer
Explanation: The enzyme catalyzed reaction which involves dissolved gases is influenced by solubility of gases, difference in molar volumes between reactants and products and volume changes occurring during binding and catalysis. In some reactions, the volume may get reduced to 50 ml/mol due to conformational changes and changes in hydration. This in turn leads to doubling of Kcat and halving of Km. In this way, changes in pressure also affects the enzyme catalyzed reaction.
9. The equation \(t_{1/2}=\frac{0.693}{k_d}\) represents ___________
a) Arrhenius equation
b) Lineweaver Burk equation
c) Half-life
d) Gibbs-Helmholtz equation
View Answer
Explanation: The above equation is representing half-life of an enzyme. Half-life of an enzyme is the time it takes for the activity to reduce to half of the original activity. Half-life is inversely proportional to rate of denaturation. Arrhenius equations describes the rate of an enzyme catalyzed reaction with an increase in temperature. Lineweaver Burk equation describes the rate of an enzyme catalyzed reaction. The relationship between the change in the pKa and the change in temperature is given by the Gibbs-Helmholtz equation.
10. If the rate of denaturation of enzyme Y is 1.5*10-4/min, then the half-life of enzyme Y is ______ mins.
a) 0.462*103
b) 4.62*103
c) 46.2*103
d) 0.0462*103
View Answer
Explanation: The half-life of an enzyme is given by, \(t_{1/2}=\frac{0.693}{k_d}\)
Where t1/2 = half-life, kd = rate of denaturation
kd = 1.5*10-4
t1/2 = 0.693/(1.5*10-4) = 0.462*104 = 4.62*103 mins.
11. What is the rate of denaturation kd, if the half-life of an enzyme catalyzed reaction is 15.6 mins?
a) 4.4*10-2/sec
b) 0.4*102/min
c) 4.4*10-2/min
d) 0.4*102/sec
View Answer
Explanation: The half-life of an enzyme is given by, \(t_{1/2}=\frac{0.693}{k_d}\)
kd = \(\frac{0.693}{t_{1/2}}\)
kd =\(\frac{0.693}{15.6}\) = .044 = 4.4*10-2/min.
12. The pH at which half the groups of a compound are ionized is referred to as _________
a) pKa
b) pI
c) I
d) Km
View Answer
Explanation: pKa is defined as the pH at which half of the groups are ionized. pKa is given by the equation, pKa = -log10[Ka], where Ka = Dissociation constant of an acid. Isoelectric pH (pI) is defined as the pH at which net charge on the molecule is zero. Ionic strength (I) is defined as half of the total sum of the concentration (ci) of every ionic species (i) in the solution times the square of its charge. Km is the substrate concentration at which half of the enzymes have bound substrate.
13. If Ka for acetic acid is 1.78*10-5, the pKa of acetic acid is _________
a) 0.475
b) 4.75
c) 2.5
d) 0.25
View Answer
Explanation: pKa is given by pKa = -log10[Ka]
pKa = -log10[1.78*10-5] = – [-4.75] = 4.75.
Sanfoundry Global Education & Learning Series – Enzyme Technology.
To practice all areas of Enzyme Technology for Freshers, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Biotechnology Books
- Check Enzyme Technology Books
- Apply for Chemical Engineering Internship
- Apply for Biotechnology Internship
- Check Chemical Engineering Books
