This set of Enzyme Technology Interview Questions and Answers for Experienced people focuses on “Enzyme Kinetics – Mechanism of Enzyme Action – 2”.
1. Which of this is not true for the active site of an enzyme?
a) Active site is constituted by 3 to 4 amino-acids in a big protein structure
b) Active site is mainly constituted by non-polar amino acids for catalysis to take place
c) Water is generally included in the site
d) Active site is a cleft formed by different amino-acids from different positions in an enzyme molecule
View Answer
Explanation: Active site has the following features:
* It is a cleft formed by different amino-acids from different positions in an enzyme molecule.
* It is constituted by 3 to 4 amino-acids in a big protein structure.
* It is mainly constituted by non-polar amino-acids for catalysis to take place. Sometime polar may also be present. Water is excluded from the site unless it’s a reactant.
* The ES complex is a must to form products. So, appropriate substrate binding site is essential for any enzyme.
* The specificity of substrate binding is mediated by spatial arrangement of atoms in an enzyme’s substrate binding site as well as substrate.
Therefore, water is generally included in the site is not true for active site.
2. Daniel Koshland proposed a model for enzyme’s reaction mechanism in 1958 which is termed as __________
a) Induced fit model
b) Lock and key model
c) Henri kinetic model
d) Miachelis Menten kinetic model
View Answer
Explanation: Henri and Michalis Menten kinetic model was proposed in 1903 and 1913 respectively. The main difference is that Miachelis and Menten did a strong experimental work to prove that enzyme substrate catalyzed reaction will possess a hyperbolic curve. Daniel Koshland proposed induced fit model. Whereas, Emil Fischer proposed lock and key model.
3. Which is the model proposed in the following diagram?
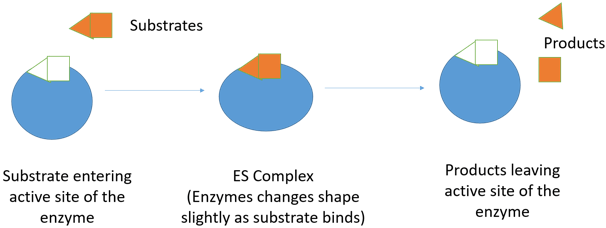
a) Fluid Mosaic Model
b) Lock and key model
c) Induced fit model
d) Miachelis Menten model
View Answer
Explanation: The diagram shows induced fit model proposed by Daniel Koshland in 1958. According to his model, an enzyme molecule induces change in its shape due to non-covalent interactions between active site and substrate. This may lead to change in 3D structure of an enzyme without affecting the primary structure and also forming ES complex that is essential for formation of products.
4. In lock and key model, the enzyme catalyzed reaction may produce an adverse reaction.
a) True
b) False
View Answer
Explanation: The lock and key model represents that as a key fits into a lock, the substrate in the same way binds to active site of the enzyme producing required products. In this model, the catalytic site is freely accessible. Thus any other compound can also interact with the active site and produce an adverse reaction. Hence the statement.
5. The energy required to attain transition state is referred to as _____________
a) Gibbs free energy
b) Activation energy
c) Standard free energy change
d) pH
View Answer
Explanation: The key role of any enzyme is to act upon specific substrate and accelerate the reaction. For fastening of any reaction, the amino-acids side chain groups of the active site should properly interact with the substrate molecule and form a transition state. For attaining a transition state, the enzyme needs certain energy which is termed as activation energy (EA).
6. The transition state cannot be achieved by using one of the following methods?
a) Substrate collision theory
b) Lock and key model
c) Acid-base catalysis
d) Covalent catalysis
View Answer
Explanation: By using lock and key model, the transition state cannot be achieved. Lock and key model proposes the fitting of substrate into the active site of the enzyme like a key fits into its lock. Substrate collision theory, acid-base catalysis and covalent catalysis are used to achieve the transition state required during enzyme catalysis.
7. Which of the following factor has a negative impact on substrate collision theory?
a) Temperature
b) Protonation or Deprotonation
c) Substrate concentration
d) Type of medium
View Answer
Explanation: Protonation and deprotonation of enzyme or substrate leads to their structural distortion which directs improper orientation to them due to which the interaction may not be accurate which may lead to negative impact. Temperature, substrate concentration and type of medium have positive impact on the substrate collision theory.
8. The type of catalysis wherein the proton donation or acceptance is done by water molecule is referred to as _____________
a) substrate collision theory
b) lock and key model
c) general acid-base catalysis
d) specific acid-base catalysis
View Answer
Explanation: In most of the enzymatic reactions, some type of proton transfer is involved. If the proton donation or acceptance is done by water molecules during catalysis is called specific acid-base catalysis. If the catalysis involves participation of small organic molecules, co-factors and amino-acids side chains from the enzyme is referred to as general acid-base catalysis. Lock and key deals with the interaction between substrate and enzyme at active site in a way as key fits into its lock. Substrate collision theory deals with collision between substrate and enzyme.
9. In the catalysis of RNA by Bovine pancreatic RNase A, His 12 acts as proton donor.
a) True
b) False
View Answer
Explanation: In most of the enzymatic reactions, some type of proton transfer is involved. In catalysis of RNA by bovine pancreatic RNase A, His 12 (imidazole) acts as proton acceptor and His 119 acts as a proton donor to attain the transition state. Hence the above statement is false.
10. The phenomenon which involves formation of an additional covalent intermediate in a reaction that helps to reduce the energy of later transition states is referred to as ___________
a) covalent catalysis
b) substrate collision theory
c) general acid-base catalysis
d) specific acid-base catalysis
View Answer
Explanation: Covalent catalysis involves substrates forming transient covalent bond with the residues present in the active site. The phenomenon which involves formation of an additional covalent intermediate in a reaction that helps to reduce the energy of later transition states is referred to as covalent catalysis. The covalent bond must be broken to regenerate the free enzyme at the later stage of the reaction.
11. Chymotrypsin: Nucleophilic catalysis:: Carboxypeptidase A: __________
a) General acid-base catalysis
b) Specific acid-base catalysis
c) Electrophilic catalysis
d) Nucleophilic catalysis
View Answer
Explanation: Chymotrypsin mediated peptide bond is an example of nucleophilic catalysis. In the same way, carboxypeptidase A is and an example of electrophilic catalysis. In most of the enzymatic reaction protons are involved. General and specific acid-base catalysis are types of acid-base catalysis.
12. A type of catalysis which involves covalent intermediate transition state formation between the cationic electrophilic group in the enzyme and the electron rich portion of the substrate is referred to as ____________
a) general acid-base catalysis
b) electrophilic catalysis
c) nucleophilic catalysis
d) specific acid-base catalysis
View Answer
Explanation: In most of the enzymatic reaction protons are involved. General and specific acid-base catalysis are types on acid-base catalysis. Nucleophilic catalysis involves donation of electrons from nucleophilic active site residues to a substrate forming a covalent adduct transition state intermediate. Whereas, electrophilic catalysis involves covalent intermediate transition state formation between the cationic electrophilic group in the enzyme and the electron rich portion of the substrate.
13. _____________ involves donation of electrons from nucleophilic active site residues to a substrate forming a covalent adduct transition state intermediate.
a) General acid-base catalysis
b) Specific acid-base catalysis
c) Electrophilic catalysis
d) Nucleophilic catalysis
View Answer
Explanation: In most of the enzymatic reaction protons are involved. General and specific acid-base catalysis are types of acid-base catalysis. The catalysis which involves donation of electrons from nucleophilic active site residues to a substrate forming a covalent adduct transition state intermediate is referred to as nucleophilic catalysis. Nucleophilic and electrophilic catalysis are used to aid covalent catalysis.
14. Which of these is not required for electrophilic catalysis?
a) Mg2+
b) Zn2+
c) Mn2+
d) Ser 195
View Answer
Explanation: Electrophilic catalysis involves covalent intermediate transition state formation between the cationic electrophilic group in the enzyme and the electron rich portion of the substrate. Most of the amino-acid side chains in the active site don’t act as an efficient electrophile. So, most of the enzymes require metal ions such as Mg2+, Zn2+, Mn2+, etc., as an electron deficient electrophile for optimum catalysis. Ser 195 is an amino-acid in the active site chymotrypsin which hydrolyses peptides.
Sanfoundry Global Education & Learning Series – Enzyme Technology.
To practice all areas of Enzyme Technology for Interviews, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Biotechnology Internship
- Check Biotechnology Books
- Practice Biotechnology MCQs
- Practice Chemical Engineering MCQs
- Check Enzyme Technology Books
