This set of Engineering Materials & Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Phases and Phase Diagrams”.
1. Which of the following is not a name for phases present in a system of material in various conditions?
a) Phase diagram
b) Equilibrium diagram
c) Interstitial diagram
d) Constitutional diagram
View Answer
Explanation: A phase diagram is a graphical representation of the phases present in the system of materials at various temperatures, pressures, and compositions. These diagrams show the constitution of alloys as a function of temperature under equilibrium conditions. They are otherwise also known as equilibrium or constitutional diagrams.
2. Which of the following cannot be obtained using a phase diagram?
a) Melting temperatures of various phases
b) Temperature range for solidification
c) Equilibrium solid solubility
d) Purity of materials
View Answer
Explanation: A phase diagram is a graphical representation of the phases present in the system of materials at various temperatures, pressures, and compositions. It can be used to determine the melting temperature of various phases, the range of solidification, and the equilibrium solid solubility of one element in another.
3. A specific body of material or a series of alloys with the same compositions is/are known as _________
a) Component
b) System
c) Alloy
d) Solute
View Answer
Explanation: A component is considered as a pure metal or compound of an alloy, whereas alloys are mixtures of two or more metals or non-metals. The system may be defined as either a specific body of material or a series of possible alloys consisting of the same components.
4. How many types of systems are applicable for phase diagrams?
a) One
b) Two
c) Three
d) Four
View Answer
Explanation: System may be defined as either a specific body of material or a series of possible alloys consisting of the same components. A system having one component is called as a unary system. Similarly, two, three, and four component systems are called binary, ternary, and quaternary systems respectively.
5. The maximum concentration of solute that can be added is defined as ____________
a) Solution limit
b) Solubility limit
c) Concentration
d) Degrees of freedom
View Answer
Explanation: Solubility limit is defined as the maximum concentration of solute that may be added without the formation of a new phase. An excess addition may result in the formation of another solid solution or compound. Degrees of freedom is defined as the number of independent variables that can be changed independently without changes in the phases of the system.
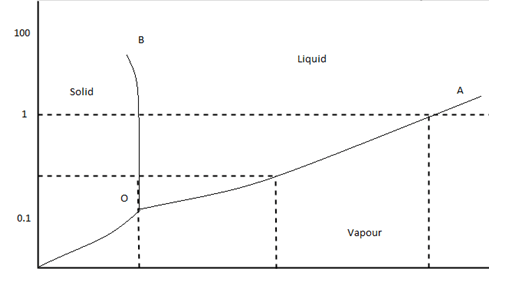
6. In this figure, what does O denote?
a) Melting point
b) Boiling point
c) Triple point
d) Vaporization point
View Answer
Explanation: This graph illustrates the pressure-temperature diagram for a one-component phase diagram of the H2O system. The point O is marked as the triple point, which is the point at which three phases exist at the same time.
7. What is the triple point of water?
a) 0.0022oC
b) 0.0098oC
c) 0.022oC
d) 0.098oC
View Answer
Explanation: The triple point is defined as the point at which three phases (liquid, solid, and vapor) exist at the same time. The triple point of water is 0.0098oC at pressure 4.58 mm of Hg.
8. How is Gibb’s phase rule defined?
a) C+P+1
b) C+P+2
c) C-P+2
d) C-P
View Answer
Explanation: The number of phases present in an alloy depends on the number of elements of which it is composed. The Gibb’s phase rule is given by the equation F = C – P + 2. Here, F is the degrees of freedom, C is the number of components, and P is the number of phases.
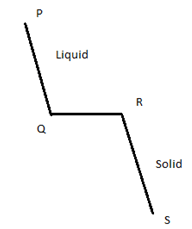
9. What is the line QR in this fooling curve known as?
a) Latent heating line
b) Eutectic compound system
c) Binary solid
d) Horizontal thermal arrest
View Answer
Explanation: Cooling curves are obtained by plotting the measured temperatures at equal intervals during the cooling period of a melt to a solid. This diagram shows the cooling curve for a pure metal or compound. PQ is a uniform curve, whereas QR is known as the horizontal thermal arrest.
10. Separation of single-phase solid regions from two-phase solid regions is done by _________
a) Solidus line
b) Liquidus line
c) Solvus line
d) Eutectic point
View Answer
Explanation: The liquidus line separates liquid and liquid+solid phase regions, whereas solidus line separates solid and solid+liquid phase regions. A solvus line separates single-phase solid regions from two-phase solid regions.
11. The point at which two liquidus lines meet is known as __ ________
a) Eutectic point
b) Isothermal point
c) Solvus point
d) Peritectic point
View Answer
Explanation: The liquidus line separates liquid and liquid+solid phase regions. Two liquidus lines meet at a point defined as the eutectic point. The corresponding temperature and composition are defined as eutectic temperature and eutectic composition.
12. Which reaction does this equation denote?
Liquid + Solid 1 → Solid 2
a) Eutectic
b) Peritectic
c) Eutectoid
d) Peritectoid
View Answer
Explanation: In peritectic reactions, a solid and a liquid, due to the action of cooling, transform isothermally and reversibly into a solid with another composition. It is also known as an upside-down eutectic reaction.
13. Which reaction does this equation denote?
Solid 1 + Solid 2 →Solid 3
a) Eutectic
b) Peritectic
c) Eutectoid
d) Peritectoid
View Answer
Explanation: In peritectoid reactions, two solid phases isothermally and reversibly transform into a solid with a third and different composition. It is also known as an upside-down eutectoid reaction. Such reactions are commonly found in Ni-Zn, Fe-Nb, Cu-Sn, and other systems.
Sanfoundry Global Education & Learning Series – Engineering Materials & Metallurgy.
To practice all areas of Engineering Materials & Metallurgy, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Engineering Materials Books
- Practice Metallurgical Engineering MCQs
- Apply for Metallurgical Engineering Internship
- Check Metallurgical Engineering Books
