This set of Engineering Materials & Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Hume Rothery’s Rules, Types of Solid Solutions”.
1. Why are Hume Rothery’s rules followed?
a) Extensive solid solution
b) Liquid solution
c) Weak solid solution
d) Extensive liquid solution
View Answer
Explanation: To form an extensive solid solution, Hume Rothery’s rules are obeyed. An extensive solid solution is generally considered as one that is greater than 10 atomic percent soluble.
2. According to Hume Rothery’s rules, size of atoms must not differ by more than ________
a) 5%
b) 15%
c) 35%
d) 55%
View Answer
Explanation: Hume Rothery’s rules state that the atomic radius or size of solute and solvent must not differ by more than 15%. This must be in order to minimize the lattice strain.
3. What is formed when the electronegativities of atoms differ?
a) Solid solutions
b) Liquid solution
c) Intermetallic compound
d) Maraging steel
View Answer
Explanation: According to Hume Rothery’s rules, the solute and solvent mist have similar electronegativities. If this difference is too large, the metals are inclined to form intermetallic compounds in place of solid solutions.
4. For interstitial solid solutions, atomic radii difference must not differ by more than ________
a) 25%
b) 37%
c) 59%
d) 73%
View Answer
Explanation: For interstitial solid solutions, Hume Rothery’s rules state that the radius of solute atoms must not be larger than solvent atoms by more than 59%. Moreover, they should have similar electronegativities and valencies.
5. Dissolution of limited amount of solute in solvent, the solution is a __________
a) Saturated solution
b) Unsaturated solution
c) Supersaturated solution
d) Oversaturated solution
View Answer
Explanation: If the solvent is dissolving a limited quantity of solute, it is known as a saturated solution. An unsaturated solution is considered one where a small quantity of solute is dissolved in a solvent, whereas for a supersaturated solution, the amount of solute in a solvent is more.
6. A solution of exchange of impurities for solvent atoms is called a _________
a) Interstitial solid solution
b) Substitutional solid solution
c) Saturated solution
d) Unsaturated solution
View Answer
Explanation: When the solute atoms (impurities) are substituted for parent solvent atoms in a crystal lattice, the atoms are called substitutional atoms. Such a mixture of two elements is called a substitutional solid solution.
7. What kind of solid solution is found in a Cu-Ni crystal?
a) Interstitial solid solution
b) Substitutional solid solution
c) Supersaturated solution
d) Unsaturated solution
View Answer
Explanation: The Cu-Ni system obeys Hume Rothery’s laws of similar atomic radii (1.28 and 1.25), same FCC crystal structure, similar valencies (+1 and +2), and similar electronegativities (1.9 and 1.8). These elements are completely soluble in one another and form a substitutional solid solution.
8. Which of the following is a random substitutional solid solution?
a) Cu-Zn
b) Au-Cu
c) Cu2MnAl
d) Carbon in ϒ iron
View Answer
Explanation: A random substitutional solid solution is one in which the solute and solvent atoms occupy random positions in the crystal lattice. Cu-Zn, or brass, is such a random substitutional solid solution.
9. Which type of solid solution does this figure illustrate?
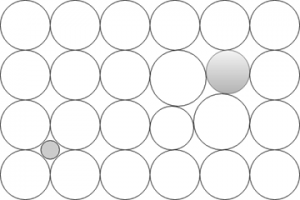
a) Interstitial solid solution
b) Substitutional solid solution
c) Supersaturated solution
d) Unsaturated solution
View Answer
Explanation: An interstitial solid solution is one which solute atoms fit into spaces between solvent atoms. These spaces are known as interstices. In the figure, a system of carbon in FCC ϒ iron is shown.
10. Au-Cn is an example of _________ solid solution.
a) Interstitial solid solution
b) Random substitutional solid solution
c) Supersaturated solution
d) Ordered substitutional solid solution
View Answer
Explanation: An ordered substitutional solid solution is one in which the solute and solvent atoms occupy specific or preferred positions in the crystal lattice. Au-Cu and Cu2MnAl are examples of such ordered substitutional solid solution.
11. Interstitial solutions have a _________ distribution.
a) Random
b) Linear
c) Alternating
d) Dendritic
View Answer
Explanation: In interstitial solutions, the solute atoms are randomly distributed throughout the solvent. In interstitial compounds, however, the pattern is regular in the specific compound.
12. Cu3Al and NiAl are examples of __________
a) Interstitial solutions
b) Interstitial compounds
c) Electron compounds
d) Valency compounds
View Answer
Explanation: If two metals consist of atoms of similar valencies, then the compounds formed are known as electron compounds. Cu3Al, CuZn, Cu3Sn and NiAl are examples of such electron compounds.
Sanfoundry Global Education & Learning Series – Engineering Materials & Metallurgy.
To practice all areas of Engineering Materials & Metallurgy, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Metallurgical Engineering Internship
- Check Engineering Materials Books
- Practice Metallurgical Engineering MCQs
- Check Metallurgical Engineering Books
