This set of Engineering Materials & Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Bonding in Solids”.
1. What is the bond energy of iron?
a) 7.8 KJ/mol
b) 401.3 KJ/mol
c) 639.5 KJ/mol
d) 1692.9 KJ/mol
View Answer
Explanation: The bond energy of a material is defined as the energy required to return the atoms to an infinite separation. Iron forms a metallic bond with a bond energy of 401.3 KJ/mol.
2. Which type of bond is formed in silicon dioxide?
a) Metallic
b) Covalent
c) Ionic
d) Intermolecular
View Answer
Explanation: The strength of the interatomic bond influences the melting and boiling points of the substances. Silicon dioxide has a covalent bond, whereas sodium chloride and nitrogen are ionic and intermolecular, respectively.
3. What is the bond energy of silicon dioxide?
a) 7.8 KJ/mol
b) 401.3 KJ/mol
c) 639.5 KJ/mol
d) 1692.9 KJ/mol
View Answer
Explanation: The bond energy of a material is defined as the energy required to return the atoms to an infinite separation. Silicon dioxide forms a covalent bond with a bond energy of 1692.9 KJ/mol. Nitrogen, iron, and sodium chloride have bond energies of 7.8 KJ/mol, 401.3 KJ/mol, and 1692.9 KJ/mol correspondingly.
4. A van der Waals bond is a _________ bond.
a) Primary
b) Secondary
c) Tertiary
d) Quaternary
View Answer
Explanation: Atomic bonds are classified as either primary bonds or secondary bonds. Primary bonds are further classified as ionic, covalent, and metallic, whereas van der Waals is a secondary bond.
5. Which bond does this representation illustrate?
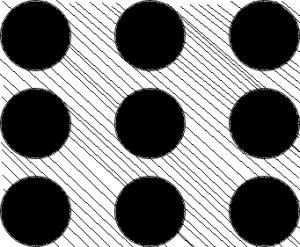
a) Metallic
b) Covalent
c) Ionic
d) Intermolecular
View Answer
Explanation: The strength of the interatomic bond influences the melting and boiling points of the substances. In metallic bonding, the valency electrons are not bound to any particular pairs of atoms. These are applied to most pure metals and alloys.
6. Covalent bond is also known as ___________ bond.
a) Electrovalent
b) Electrolytic
c) Heteropolar
d) Homopolar
View Answer
Explanation: Covalent bonds are primary bonds which are formed when electrons are exchanged between atoms. It is also called as a homopolar bond. An ionic bond can also be called a heteropolar of the electrovalent bond.
7. Which among the following is not a type of intermolecular bond?
a) Dispersion
b) Dipole
c) Nitrogen
d) Hydrogen
View Answer
Explanation: Intermolecular forces are weak forces that account for the mutual interaction between the molecules. These intermolecular bonds are classified as dispersion bonds, hydrogen bonds, and dipole bonds.
8. Which type of bonding does this illustration depict?
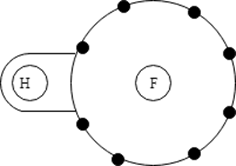
a) Dispersion
b) Dipole
c) Ionic
d) Hydrogen
View Answer
Explanation: The center of positive charge and negative charge are separated. This produces an electrical dipole which provides a mechanism for molecular bonding.
9. How strong is a dipole bond?
a) Stronger than ionic bond
b) Stronger than dispersion bind
c) Weaker than dispersion bond
d) Equal to ionic bond
View Answer
Explanation: An electrical dipole provides a mechanism for molecular bonding. The presence of a permanent dipole moment increases the attraction forces between molecules. A dipole bond is weaker than an ionic bond but is stronger than a dispersion bond.
Sanfoundry Global Education & Learning Series – Engineering Materials & Metallurgy.
To practice all areas of Engineering Materials & Metallurgy, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Metallurgical Engineering Internship
- Practice Metallurgical Engineering MCQs
- Check Metallurgical Engineering Books
- Check Engineering Materials Books
