This set of Materials Science Multiple Choice Questions & Answers (MCQs) focuses on “Gibbs Phase Rule”.
1. What is Gibbs phase rule for general system?
a) P = C – 1 – F
b) P = C + 1 – F
c) P + F = C – 2
d) P + F = C + 2
View Answer
Explanation: The number of degrees of freedom, F (no. of independently variable factors), number of components, C, and number of phases in equilibrium, P.
2. What is Gibbs phase rule for metallurgical system?
a) F = C – 1 – P
b) F = C + 1 – P
c) P + F = C – 2
d) P + F = C + 2
View Answer
Explanation: For metallurgical system pressure has no appreciable effect on phase equilibrium and hence, F = C – P + 1.
3. In a single – component condensed system, if degree of freedom is zero, maximum number of phases that can co – exist _________
a) 2
b) 3
c) 0
d) 1
View Answer
Explanation: Given F = 0
Then p = c + 1, c = 1
.: P = 2.
4. The degree of freedom at a triple point in the unary diagram for water is ________
a) 2
b) 3
c) 0
d) 1
View Answer
Explanation: For three phase system degree of freedom is 0.
5. What is degree of freedom for single – phase fields on the phase diagram?
a) 2
b) 3
c) 0
d) 1
View Answer
Explanation: F = C + 1 – P
F = 3 – P (C = 2)
.:F = 2.
6. What is degree of freedom when two phases co – exist?
a) 2
b) 3
c) 0
d) 1
View Answer
Explanation: F = C + 1 – P
F = 3 – P (C = 2)
F = 3 – 2 = 1.
7. What is degree of freedom when three phases co – exist?
a) 2
b) 3
c) 0
d) 1
View Answer
Explanation: F = C + 1 – P
F = 3 – P (C = 2)
F = 3 – 3 = 0.
8. For single component system when degree of freedom is 1(one) then number of phases _______
a) 2
b) 3
c) 0
d) 1
View Answer
Explanation: F = C + 1 – P
F = 2 – P (C = 1)
→ p = 2 – F = 2 – 1 = 1.
9. When α, L and β phase fields touch the isotherm line what are the respective phase compositions?
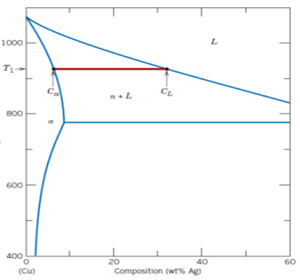
a) 8.0 wt%, 71.9 wt%, 91.2 wt% of Ag
b) 8.0 wt%, 91.2 wt%, 71.9 wt% of Ag
c) 71.9 wt%, 91.2 wt%, 8.0 wt% of Ag
d) 91.2 wt%, 8.0 wt%, 71.9 wt% of Ag
View Answer
Explanation: For binary systems, when three phases are present, there will be F = 0, so composition is fixed.
10. For binary alloy consisting of three phases of non – equilibrium one, What will be the temperature of these phases?

a) Different
b) Constant
c) Same
d) Two of them will be with one temperature
View Answer
Explanation: One use of the Gibbs phase rule is in analyzing for non – equilibrium conditions by analyzing with above method we come to know (under these Circumstances, three phases will exist only at a single temperature).
Sanfoundry Global Education & Learning Series – Materials Science.
To practice all areas of Materials Science, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
