This set of Materials Science Quiz focuses on “Basic Concepts of Phase Diagrams”.
1. Which of the following is/are the components of a brass alloy?
a) Brass, Copper, Zinc
b) Copper only
c) Zinc only
d) Both Copper and Zinc
View Answer
Explanation: Components are pure metals and/or compounds of which an alloy is composed. Brass is an alloy and Copper and zinc are components.
2. At certain temperature the maximum concentration of solute atoms that dissolve in solvent to form solid solution, this condition is called __________
a) Solubility
b) Formation of phase
c) Solubility limit
d) Formation of super saturated solution
View Answer
Explanation: At this limit there will maximum solubility o solution and solution formed is saturated above this there will be formation of super saturated solution.
3. Analyze the following graph and the following question regarding solubility of sugar in water.
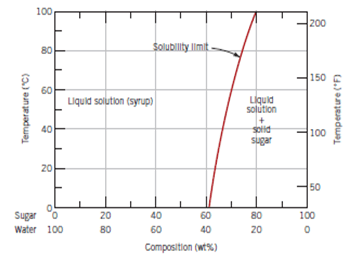
a) Decrease with increase in temperature
b) Increase with increase in temperature
c) Increase with decrease in temperature
d) Remains constant with increase or decrease in temperature
View Answer
Explanation: The solubility of sugar increases when the solution is heated.
4. What is a phase?
a) The substance which is physically distinct
b) The substance which is homogenous chemically
c) The substance which is both physically distinct and chemically homogenous
d) The substance which is both physically distinct and chemically heterogeneous
View Answer
Explanation: A phase can be defined as a physically distinct and homogeneous chemically portion of a system that has a particular chemical composition and structure.
5. Which of the following portion(s) of the given diagram contains only gas?
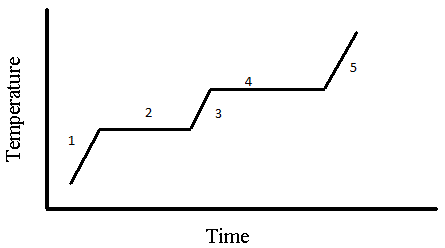
a) 1
b) 4
c) 5
d) 4, 5
View Answer
Explanation: In the figure pure substances exists at 1, 3, 5 out of which 1 is given solid so 5 is pure gas.
6. Which of the following portion(s) of the given diagram contains liquid in it?

a) 2, 3
b) 2, 4
c) 3, 4
d) 2, 3, 4
View Answer
Explanation: From figure in process 2 the substance solid is being converted to liquid. In 3 only pure liquid exists. In 4 liquid is converting into solid. These all stages contain liquid in it.
7. Which of the following portion(s) of given diagrams contains only pure substances?

a) 1, 3, 5
b) 1, 5
c) only 1
d) 3, 5
View Answer
Explanation: Pure substances are substances that contain only a single phase such as solid or liquid or gas. These phases exist in 1, 3 and 5 portions.
.
8. Which of the following portion(s) of the given diagram has a freezing section?

a) 1
b) 2
c) 3
d) 4
View Answer
Explanation: Since this is a warming curve, this section indicates the melting temperature, but freezing point is identical to melting temperature. It can be noted as freezing range in a cooling curve.
9. Which of the following portion(s) of the given graph has a boiling point?

a) 2
b) 3
c) 4
d) 5
View Answer
Explanation: At this point the substance gets converted from liquid to gas.
10. Which of the following section(s) given graph in which we use (Specific Heat) * (Mass) * (Change in temperature) to calculate the energy change?

a) 2
b) 1, 3
c) 1, 3, 5
d) 2, 3, 5
View Answer
Explanation: Since the phase of substance remains constant and there is change in temperature of substance.
Sanfoundry Global Education & Learning Series – Materials Science.
To practice all areas of Materials Science for Quizzes, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
