This set of Engineering Materials & Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Crystal Defects”.
1. Which of the following is a point defect in crystals?
a) Edge dislocation
b) Interstitialcies
c) Grain boundaries
d) Cracks
View Answer
Explanation: Crystal defects are classified as point defects, line defects, and boundary defects. Point defects include vacancies, impurities, interstitialcies, and electronic defects.
2. How can the number of defects be determined?
a) ![]()
b) ![]()
c) ![]()
d) ![]()
View Answer
Explanation: The number of defects at equilibrium at a definite temperature can be determined using the equation Ne(-Ed⁄kT). It is denoted by nd. Here, N stands for the total number of atomic spots and Ed is the activation energy.
3. The defect that occurs due to a displacement of an ion is known as __________
a) Vacancy defect
b) Schottky defect
c) Frankel defect
d) Interstitial defect
View Answer
Explanation: Frankel defect occurs due to a displacement of an ion from the crystal lattice. It is related to the interstitial defect, where an ion simply occupies a position between regular atoms.
4. Which defect does the following figure depict?
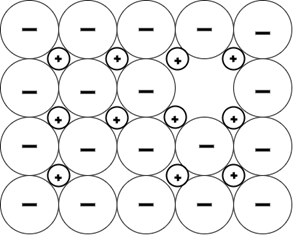
a) Vacancy defect
b) Schottky defect
c) Frankel defect
d) Interstitial defect
View Answer
Explanation: When a pair of positive and negative ions both disappear from a crystal lattice, the effect is called a Schottky defect. It is closely related to vacancy defects where simply an ion is missing.
5. Which of these is a Frankel defect?
a) 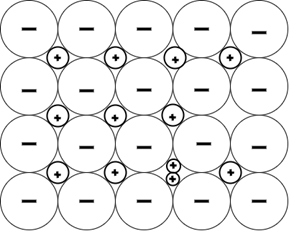
b) 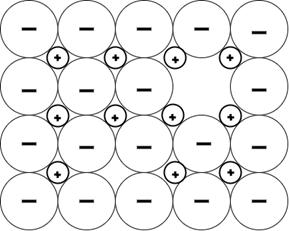
c) 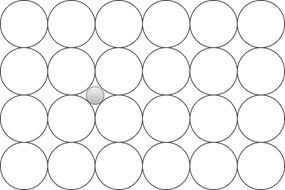
d) 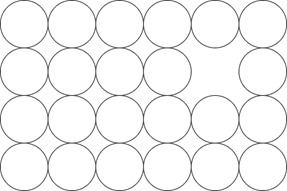
View Answer
Explanation: Frankel defect occurs due to a displacement of an ion from the crystal lattice while retaining all of the ions, unlike other defects. It is related to the interstitial defect, where an ion simply occupies a position between regular atoms.
6. Which defect does the following diagram represent?
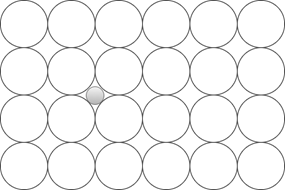
a) Vacancy defect
b) Schottky defect
c) Frankel defect
d) Interstitial defect
View Answer
Explanation: Interstitial defects occur when an atom occupies an empty position in a crystal lattice. Self-interstitial effects occur due to their own atoms, while others occur due to a foreign substance.
7. _______ occurs when a foreign substance replaces an atom in a crystal.
a) Vacancy defect
b) Substitutional impurity
c) Frankel defect
d) Interstitial impurity
View Answer
Explanation: A substitutional impurity occurs due to the occupation of a foreign atom in place of an atom in a crystal. On the other hand, interstitial impurities occur when a regular atom occupies a random space in the crystal lattice.
8. A disturbance in a region between two ideal parts of a crystal is known as ________
a) Boundary defect
b) Point defect
c) Line defect
d) Volume defect
View Answer
Explanation: Line defect is regarded as a disturbed region between two perfect parts of a crystal. They may be of either edge dislocation type or screw dislocation.
9. In screw dislocation, the Burger’s vector lies _________ to the dislocation line.
a) Perpendicular
b) Parallel
c) At an angle
d) Sideways
View Answer
Explanation: The Burger’s vector in screw dislocation lies parallel to the dislocation line along the axis of a line of atoms in the same plane. On the other hand, it lies at an angle for edge dislocation.
10. Generation of dislocations can be identified using _______
a) Schottky mechanism
b) Burger’s vector
c) Twist
d) Frank-Read mechanism
View Answer
Explanation: The Frank-Read mechanism uses the Frank-Read source and its operation as a dislocation multiplier. The Frank-Read source contains a fixed lined at the X and Y nodes. Under the application of stress, the dislocation line expands and is further operated until it becomes readable.
11. What are one-dimensional defects?
a) Boundary defect
b) Point defect
c) Line defect
d) Volume defect
View Answer
Explanation: When compared geometrically, line defects are seen as one-dimensional defects. Line defects are also known as dislocations, with common types as edge and screw dislocations.
12. What are two-dimensional defects?
a) Boundary defect
b) Point defect
c) Line defect
d) Volume defect
View Answer
Explanation: The defects that occur on the surface of a material are known as surface or boundary defects. Geometrically, they are regarded as two-dimensional defects.
13. How is the dislocation energy defined?
a) J m-1
b) J m-2
c) m-2
d) N m-1
View Answer
Explanation: Dislocation energy is defined ad joule per meter and is denoted by E. Dislocation density is defined as meter per cubic meter or simply as per meter square.
Sanfoundry Global Education & Learning Series – Engineering Materials & Metallurgy.
To practice all areas of Engineering Materials & Metallurgy, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Engineering Materials Books
- Check Metallurgical Engineering Books
- Apply for Metallurgical Engineering Internship
- Practice Metallurgical Engineering MCQs
