This set of Engineering Materials & Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Defects and Machining of Metals, Solidification of Metals”.
1. Which defect does the following image denote?
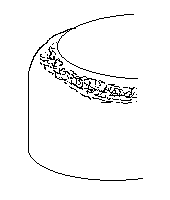
a) Orange peel
b) Sinking
c) Burr and bend
d) Wrinkling
View Answer
Explanation: Whenever a coarse grain material blank is drawn, they will often be seen as a rough surface. The metal surface experiences roughness like that or an orange peel. This is known as the orange peel effect.
2. Which defect is illustrated in the below figure?
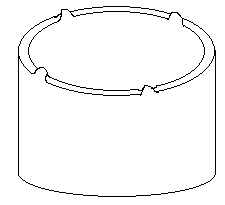
a) Earing
b) Sinking
c) Burr and bend
d) Strain hardening
View Answer
Explanation: Cold working causes uneven deformation of the material, leading to the formation of ears. To minimize earing, excessive deformation in deep drawing must be avoided.
3. Which of the following is a chipless machining method?
a) Broaching
b) Reaming
c) Abrasive machining
d) Electrical discharge
View Answer
Explanation: Chip machining forms chips due to the action of the tool being inserted into the work piece. The common chip-type machining processes are drilling, boring, planning, reaming, etc. In chipless machining, the material is removed by chemical, electrochemical, or erosion method.
4. Wrinkling is caused due to _______
a) Compressive stress
b) Tensile stress
c) Improper clearance
d) Excessive deformation
View Answer
Explanation: Wrinkling is a sheet metal defect caused due to buckling under compressive stresses. It may also occur between a punch and a blank holder.
5. Which of the following is not a stage of volume shrinkage?
a) Liquid contraction
b) Solid contraction
c) Gaseous contraction
d) Solidification contraction
View Answer
Explanation: Liquid contraction occurs when the metal is in a liquid state, whereas solid contraction occurs when the metal is solid and takes place after solidification. Solidification contraction occurs during the transition from liquid to solid state.
6. Seed crystals are formed due to _________ of melt.
a) Heating
b) Cooling
c) Overheating
d) Undercooling
View Answer
Explanation: A metal in molten state exhibits high energy. When this melt cools, it loses energy and forms crystals. When there are no nuclei to start crystallization, melt undercools and forms nuclei or seed crystals.
7. Crystal growth occurs in __________ manner.
a) Dendritic
b) Pyramidal
c) Granular
d) Linear
View Answer
Explanation: The crystal growth during solidification occurs in a dendritic style. Dendritic growth is caused by the development of small arms on branches of dendrites.
8. Which of the following holds true for pure metals?
a) Low ductility
b) High tensile strength
c) High yield point
d) Corrosion resistance
View Answer
Explanation: Pure metals like copper and aluminum generally possess excellent thermal and electrical conductivity. They also have higher ductility and corrosion resistance. However, they possess low tensile strength and yield point.
9. Pure metals generally solidify at _________ temperature.
a) Boiling
b) Freezing
c) Room
d) Cryogenic
View Answer
Explanation: Pure metals melt and solidify at the melting point or freezing temperature. The metal is in a liquid state above freezing point but is in the solid state below it.
Sanfoundry Global Education & Learning Series – Engineering Materials & Metallurgy.
To practice all areas of Engineering Materials & Metallurgy, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Metallurgical Engineering Books
- Check Engineering Materials Books
- Practice Metallurgical Engineering MCQs
- Apply for Metallurgical Engineering Internship
