This set of Engineering Materials & Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Polymers and Polymerization, Polymer Additives”.
1. Which of the following is not a characteristic trait of polymer materials?
a) Low density
b) Resistant to chemical attack
c) Low cost
d) High strength
View Answer
Explanation: Polymers are a large group of repeating units of small molecules. They have a low density and cost, with good thermal and electrical insulation. They are also easy to fabricate and are resistant to chemical attack. Their low strength and stiffness can be improved by fiber reinforcement.
2. The number of repeating units in a polymer is known as __________
a) monomer
b) degree of polymerization
c) molecule
d) chain
View Answer
Explanation: Monomers are small molecules which combine repeatedly to form a polymer. The number of these repetitive units in one molecule is referred to as the degree of polymerization. It is also known as D.P. value.
3. A polymer made of identical monomer units is called ______
a) Homopolymer
b) Linear polymer
c) Copolymer
d) Branched polymer
View Answer
Explanation: When all the monomer units in a chain are of the same type, they are called homopolymers. When a polymer is created using different types of monomers, it is a copolymer. For example, -M-M-M-M- is a homopolymer and -M1-M2-M1-M2- is a copolymer. Linear and branched polymers are types of homopolymers.
4. Liquid or gas polymers having short chains and low molecular weights are known as _____
a) High-polymers
b) Homopolymers
c) Copolymers
d) Oligo-polymers
View Answer
Explanation: Oligo-polymers are available in liquid or gas forms which have very short chains. They have a low molecular weight of the order of 100 g/mol. High-polymers have high molecular weights between 10000 to 1000000 g/mol.
5. Which molecular structure does the below figure represent?
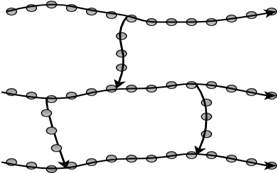
a) Linear
b) Branched
c) Cross-linked
d) Network
View Answer
Explanation: Cross-linked polymers are a type of homopolymers. They are formed when linear chains are joined together at various points. This is done due to the presence of covalent bonds.
6. Which molecular structure does the figure represent?
![]()
a) Random
b) Alternating
c) Block
d) Graft
View Answer
Explanation: Block copolymers are those containing identical monomers in blocks along the chain. Each block may contain equal to or more than two similar molecules. These blocks alternate between the two different types of monomers.
7. Which figure represents the branched molecular structure?
a) 
b) 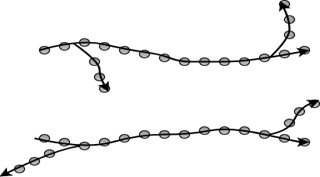
c) 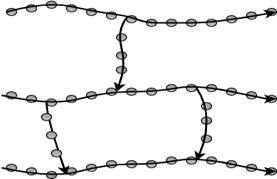
d) 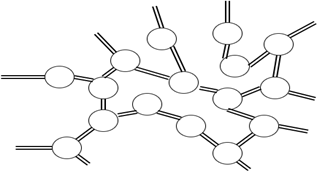
View Answer
Explanation: Branched polymers are those in which branches of monomeric chains are connected to the main chain. Linear polymers are single line chains, whereas cross-linked polymers are joined from one chain to another. Network polymers are three-dimensional chains of monomeric units.
8. Which of the following figures represent a Graft copolymer structure?
a) 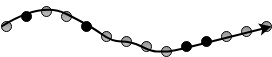
b) ![]()
c) ![]()
d) 
View Answer
Explanation: In graft copolymers, side branches of homopolymers are connected to the main chain. The side branches and main chain are composed of different monomers. Random copolymers have different monomers in random positions, while alternating polymers have different monomers in alternate places. Block copolymer is a set of blocks of different monomeric units.
9. Which of the following types of polymers is a copolymer?
a) Graft
b) Network
c) Linear
d) Branched
View Answer
Explanation: A graft polymer is a copolymer type in which the side branches of homopolymers are connected to the main chain. Random, alternating, and block are the other types of copolymers. Homopolymers exist as linear, branched, cross-linked, and network type.
10. Which of the following is not a stage of addition polymerization?
a) Initiation
b) Propagation
c) Termination
d) Recrystallisation
View Answer
Explanation: Addition polymerization is a process by which similar monomers are combined to form long chain molecules. It involves initiation, propagation, and termination. This process is also known as a chain reaction polymerization.
11. Addition of different types of monomers to form polymer chains is known as _______
a) Chain reaction polymerization
b) Copolymerization
c) Combination
d) Disproportionation
View Answer
Explanation: Copolymerization is a type of addition polymerization involving different monomers. Copolymers are widely used in plastics. For example, styrene and butadiene combine to form a copolymer.
12. What is the rate of growth of chains in condensation polymerization?
a) 10-2 to 102 seconds
b) 10 minutes
c) Hours or days
d) Weeks
View Answer
Explanation: Condensation polymerization results in the production of tri-functional monomers. These generally take a larger time for condensation; say a few hours to a few days. Addition polymerization takes 10-2 to 102 seconds for chain growth.
13. Wood flour and silica flour are examples of ______
a) Fillers
b) Plasticizers
c) Stabilizers
d) Lubricants
View Answer
Explanation: Polymer additives are used to improve the properties and performance of polymers. Fillers are used for improving strength, stability, and reducing the overall cost A few examples of fillers are wood flour, silica flour, glass, sand, mica, etc.
14. Which polymer additives are added to improve flexibility?
a) Lubricants
b) Plasticizers
c) Stabilizers
d) Reinforcements
View Answer
Explanation: Plasticizers are polymer additives which improve flexibility, ductility, and toughness of a polymer. They reduce hardness and stiffness of the polymer and increase the flow during the molding operation. Commonly used plasticizers are polyvinyl chloride and acetate copolymers.
15. Which of the following is an example of a stabilizer?
a) Benzophenon
b) Polyvinyl chloride
c) Titanium dioxide
d) Antimony oxide
View Answer
Explanation: Stabilizers in polymers help prevent deterioration of quality due to environmental and radiation effects. Hindered amines, hydroxyl phenyl benzotriazoles, and benzophenons are examples of commonly used stabilizers.
16. The optical opacity of a polymer can be controlled by using ______
a) Stabilizers
b) Colorants
c) Flame retardants
d) Reinforcements
View Answer
Explanation: Colorants are polymer additives which give the polymer a definite color and manage its optical opacity. They are also known as coloring agents or pigments. Metal oxides, sulfur diodes, and titanium dioxides are a few examples of colorants.
17. Which of the following is an example of flame retardants?
a) Talc
b) Dye
c) Antimony oxide
d) Glyceride
View Answer
Explanation: Flame retardants are used to suppress the flammability of a polymer. Common examples of flame retardants are antimony oxide, borates, bromates, and phosphates. Talc is used as a filler whereas dye is used as a colorant. Glyceride finds its application as a lubricant.
18. Which polymer additive is used to remove parts from molds?
a) Plasticizers
b) Stabilizers
c) Lubricants
d) Reinforcements
View Answer
Explanation: Lubricants are used to remove parts from molds, make surfaces slippery, and prevent them from sticking to each other. They are also known as slip agents. Common lubricant additives are silicone, waxes, fatty acid amides, glycerides, petrolatum etc.
19. Carbon fiber is an example of ________
a) Filler
b) Stabilizer
c) Reinforcement
d) Flame retardant
View Answer
Explanation: Reinforcement is a type of polymer additive which is used to improve the strength and rigidity of the polymer. Common examples of reinforcements are carbon fiber, glass fiber, fabrics, and mica. Mica is, sometimes, also used as a filler additive.
Sanfoundry Global Education & Learning Series – Engineering Materials & Metallurgy.
To practice all areas of Engineering Materials & Metallurgy, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Metallurgical Engineering MCQs
- Check Engineering Materials Books
- Apply for Metallurgical Engineering Internship
- Check Metallurgical Engineering Books
