This set of Engineering Materials & Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Refractory Materials, Superalloys”.
1. Which type of refractory brick does the following illustration represent?
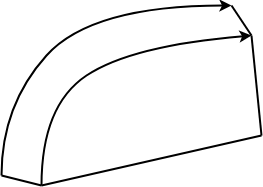
a) Wedge
b) End Skew
c) Jamb
d) Soap
View Answer
Explanation: Refractory bricks are formed after processes such as mining, calcining, crushing, and grinding. This is then mixed with other materials and the desired brick is formed. The above picture illustrates a jamb brick, whereas a soap brick appears as a long cuboidal box.
2. How much silica do silica refractories usually contain?
a) 95 – 97%
b) 0.2 – 1.0%
c) 1.8 – 3.5%
d) 0.3 – 0.9%
View Answer
Explanation: Silica is a common type of acid refractory material containing high amounts of silica and low amount of alumina. It contains about 95 – 97% of SiO2, 0.2 – 1.0% of Al2O3, 1.8 – 3.5% of CaO, and 0.3 – 0.9% of Fe2O3. It has an approximate fusion temperature of 1700oC.
3. Which of the following is a property of fireclay?
a) Rigid under load
b) Poor resistance to spalling
c) Instability in volume
d) Poor resistance to attack by alkalies
View Answer
Explanation: Fireclay is a common refractory brick which is available as a semi-silica class. It has a good rigidity under load at high temperatures and good resistance to structural spalling. These bricks are also resistant to penetration and attack by alkalies and fumes, and also possess volume stability.
4. How is the corrosion resistance of high alumina when compared against fireclay?
a) Higher
b) Equal
c) Lower
d) No resistance
View Answer
Explanation: High alumina is available with 50-90% alumina. It has great mechanical strength at higher temperatures and good resistance to spalling. It possesses greater resistance to corrosion against fireclay bricks.
5. What is the hardness of tungsten at room temperature?
a) 250 VHN
b) 480 VHN
c) 155 VHN
d) 60 VHN
View Answer
Explanation: Tungsten is a common refractory material which has the highest melting point of 3410oC among metals. It also has a hardness of 480 (VHN) on the Vickers scale. The hardness of molybdenum and tantalum is 250 VHN and 155 VHN respectively.
6. TMZ is an alloy of _______
a) Tungsten
b) Magnesium
c) Molybdenum
d) Tin
View Answer
Explanation: TMZ is a molybdenum base alloy which is frequently used due to its availability and machinability. It consists of 0.5% titanium, 0.08% zirconium, and remaining molybdenum. These alloys are used for die casting, extrusion, and forging dies.
7. Which of the following is not a characteristic of tungsten?
a) Good strength
b) Good electrical conductivity
c) Heavy
d) Good insulation
View Answer
Explanation: Tungsten is a refractory metal having the same density as gold, is heavy, and has the highest melting point among metals. It possesses a good strength under high temperature and also good electrical conductivity. It is generally used in lamps, electron tubes, and other electrical parts.
8. How can the ductility of tungsten be improved?
a) Cold working
b) Condensation
c) Hot working
d) Forming
View Answer
Explanation: Due to the nature of tungsten, it has a low ductility and malleability at room temperature. Its ductility can be enhanced by lowering the recrystallization temperature. This can be achieved by cold working, which lowers the temperature from 600oC to 190oC.
9. What is the oxidizing temperature of niobium?
a) 100oC
b) 200oC
c) 400oC
d) 600oC
View Answer
Explanation: Niobium is a common nuclear and aerospace material which is often found and used with tantalum. Oxidization of niobium occurs above 400oC. To prevent this oxidization, a protective coating is applied to the material to keep it from becoming too brittle.
10. Which phase represents the matrix of the alloy?
a) Gamma
b) Gamma Prime
c) Gamma Double Prime
d) Carbide
View Answer
Explanation: Gamma is that phase of a nickel-based superalloy which is composed of the matrix. It is a solid solution having an FCC structure. The commonly used nickel-based alloys are chromium, molybdenum, iron etc.
11. Which crystal structure is the Gamma Double Prime phase of nickel-based superalloy composed of?
a) FCC
b) BCC
c) BCT
d) HCP
View Answer
Explanation: Gamma Double Prime is a phase of nickel-based superalloys which are used to strengthen the alloys at temperatures lower than 650oC. It consists of a body-centered tetragonal (BCT) crystal structure having a composition of Ni3Nb or Ni3V.
12. In which temperature range is Gamma Prime phase of nickel-based superalloys unstable?
a) 100-200oC
b) 200-400oC
c) 600-850oC
d) 850-1000oC
View Answer
Explanation: The Gamma Prime phase of nickel-based superalloys is used to strengthen the alloys at temperatures lower than 650oC. It is an intermetallic phase consisting of an FCC crystal structure. They are unstable between 600-850oC due to which a transformation from FCC structure into HCP occurs.
13. TCP phase of nickel-based superalloys are formed at a temperature of _______
a) 200oC
b) 400oC
c) 600oC
d) > 750oC
View Answer
Explanation: TCP refers to a Topologically Close-Packed phase in which the phases have close-packed planes. It includes several phases existing in HCP crystal structure. This phase is formed as a result of high temperature over a long period of time.
14. Which of the following is not an effect of adding boron and silicon to superalloys?
a) Improves adhesion
b) Maintains oxide layer
c) Increases spalling
d) Reduces spalling
View Answer
Explanation: Since superalloys are operated at high temperatures, they are susceptible to degradation of quality. Addition of elements like boron, silicon, and yttrium improves adhesion, reduces spalling, and maintains the protective oxide layer. This is a minor form of degradation which can be corrected by coatings.
15. The thermal coating ‘thermally grown oxide’ is formed by the oxidation of _______
a) Silicon
b) Magnesium
c) Molybdenum
d) Aluminum
View Answer
Explanation: Thermal barrier coatings (TBC) are used to improve the life and performance of the component by applying a thin coating. This consists of a bond coat, thermally grown oxide, and another thermal insulation coating. This thermally grown oxide (TGO) is formed due to the oxidation of aluminum contained in a bond coat.
16. Which of the following applications does a tungsten-carbide coating provide?
a) Abrasion resistance
b) Corrosion resistance
c) Loss of coating mass
d) Heat resistance
View Answer
Explanation: Cobalt-cermet based coatings are generally used due to their effects on temperature and oxidation. Tungsten carbide is one such cobalt-cermet based coating which is resistant to abrasion, corrosion, and heat. Additionally, these coatings have the benefit of nominal loss of coating mass due to the presence of carbides.
Sanfoundry Global Education & Learning Series – Engineering Materials & Metallurgy.
To practice all areas of Engineering Materials & Metallurgy, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Engineering Materials Books
- Check Metallurgical Engineering Books
- Apply for Metallurgical Engineering Internship
- Practice Metallurgical Engineering MCQs
