This set of Phase Transformation Multiple Choice Questions & Answers (MCQs) focuses on “Binary Phase Diagrams”.
1. Which among the following condition about a simple binary system is not a part of Hume- Rothery rule?
a) Same crystal structure
b) Size difference is less than 15
c) Electronegativity’s have similar values
d) Composition should be almost similar
View Answer
Explanation: Hume-Rothery rules doesn’t state anything about the composition of the system. Hume-Rothery rule states about the atomic size factor, it states about the similarity of 2 elements based on the solid solubility factor, it tells about the dissolving capacity of a metal based on its valency and it relates the electronegativity with solubility.
2. The Gibbs free energy variation for a binary system of constituents A and B is shown in the figure below. The point of intersection corresponds to____________________
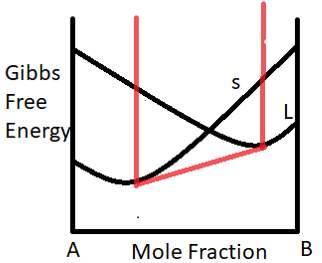
a) The composition of the solid particle that precipitate out
b) The composition of the liquid particle that precipitate out
c) The amount of solid that converts to liquid
d) The amount of liquid that converts to solid
View Answer
Explanation: For the situation corresponding to the point of intersection of the solidus and liquid line indicate the composition of the solid particles that precipitate out at the initiation of the solid liquid transformation during cooling.
3. Enthalpy of mixing is negative, for such systems melting will be more difficult in the alloys and a maximum melting point mixture may appear.
a) True
b) False
View Answer
Explanation: In these systems melting will be more difficult in the alloys and a maximum melting point mixture may appear. This type of alloy also has a tendency to order at low temperatures. If the attraction between unlike atoms is very strong the ordered phase may extend as far as the liquid.
4. What happens when the the atoms in the alloy ‘repel’ each other making the disruption of the solid into a liquid phase possible at lower temperatures than in either pure A or pure B?
a) Alloys melt at temperature below melting point
b) Alloys melt at temperature above melting point
c) Alloys melt at room temperature
d) Alloys solidify
View Answer
Explanation: The effect of a positive enthalpy of mixing in the solid is already apparent at higher temperatures where it gives rise to a minimum melting point mixture so hence the alloys melt at temperature below the melting point.
5. The stable composition range of the phase in the phase diagram need not include the composition with the minimum free energy, but is determined by _____
a) Relative free energy of adjacent phases
b) Enthalpy of phases
c) Free energy of stable phase
d) Relative enthalpy of adjacent phases
View Answer
Explanation: The Relative free energy of adjacent phases is used to determine the composition with minimum energy, actually this is an interesting result of common tangent construction.
6. According to Gibbs rule the number of phases (P) present in a system in equilibrium is given as_________
a) P=C+N-F
b) P=C+F-N
c) P=F+N-C
d) P=C+N+F
View Answer
Explanation: P+F=C+N, where F is the degree of freedom, N is the non-compositional variable and C is the number of components. Here the degree of freedom is an intensive variable hence can be varied independently while still maintaining the equilibrium.
7. The equations for free energy and chemical potential can be used to derive the effect of temperature on the limits of solid solubility in a terminal solid solution.
a) True
b) False
View Answer
Explanation: The equations for free energy and chemical potential can be used to derive the effects and this can be proved by using a phase diagram and from the equation dG= -SdT + VdP + μdN, it is clear that the free energy changes with the change in pressure, temperature and chemical potential.
8. What happens to the internal energy when atoms are moved from their respective sites?
a) Internal energy remains constant
b) Internal energy increases
c) Internal energy decreases first then increases
d) Internal energy decreases
View Answer
Explanation: The removal of atoms from their sites increases the internal energy of the metal because atoms at their respective positions are stable. If they are disturbed, forced, or moved from their respective sites then due to the broken bonds around the vacancy the internal energy increases.
9. What happens to the configurational entropy when atoms are moved from their respective sites?
a) Remains constant
b) Decreases
c) Increases
d) Become zero
View Answer
Explanation: Due to the increase in randomness the configurational entropy increases. These configurational entropy can experience some changes during the ordering (Chemical) or during the magnetic phase transitions and they are difficult to calculate when there are partial correlation over small distances.
10. What causes the increase in thermal entropy due to the vacancies present in the site?
a) Collision of the adjacent atoms
b) Extra freedom of vibration
c) Due to restriction in motion
d) Due to close packing of remaining atoms
View Answer
Explanation: Energies of electrons in atoms depend upon temperature of the host material. More temperature means high amplitude atomic vibrations. More electrons will be in excited/ionized states at a higher temperature. One can notice a variation in the pattern of vibration next to the vacancies and this happens because of the extra free space.
11. In a cooling curve, the invariant reaction is represented by ______
a) Thermal arrest
b) Vertical arrest
c) Horizontal arrest
d) Slope
View Answer
Explanation: Cooling curve is a kind of line graph that depict the phase change in matter. When you fit the thermocouple in a work piece or test probe, finally you can end up obtaining a cooling curve. Horizontal arrest represents the invariant reaction in a cooling curve.
Sanfoundry Global Education & Learning Series – Phase Transformation.
To practice all areas of Phase Transformation, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Metallurgical Engineering Internship
- Check Mechanical Engineering Books
- Check Phase Transformation Books
- Check Metallurgical Engineering Books
- Apply for Mechanical Engineering Internship
