This set of Engineering Materials and Metallurgy Multiple Choice Questions & Answers focuses on “Abrasives, Silicate Structures and Polymorphism”.
1. Which of the following is not a natural abrasive?
a) Emery
b) Quartz
c) Silicon carbide
d) Sandstone
View Answer
Explanation: An abrasive is a common ceramic material which is hard and mechanically resistant. Synthetic abrasives are preferred as they can be made as per the desired properties and also exhibit more uniformity. Diamond, sandstone, quartz, and emery are a few examples of natural abrasives. Silicon carbide and aluminum oxide are examples of synthetic abrasives.
2. What is a common application of diamond?
a) Drilling of rocks
b) Sandpaper
c) Wood finishing
d) Polishing of metals
View Answer
Explanation: Diamond is a natural abrasive and is known as the hardest material. It is used for wire drawing dies, drilling rocks, and dressing grinding wheels. It is also used for polishing of hard carbide metals, glass, and ceramics. Emery, on the other hand, is used for general purpose metal polishing.
3. Sandpaper is a common application of ______ abrasives.
a) Garnet and flint
b) Rottenstone and pumica
c) Walnut shells
d) Silicon carbide
View Answer
Explanation: Garnet and flint are natural abrasives typically used for sandpaper. Walnut shells find their application for cleaning of aircraft engine parts, whereas rottenstone and pumica are used for wood finishing.
4. Silicic acid deposits are used to form _____
a) Quartz
b) Emery
c) Tripoli
d) Diamond
View Answer
Explanation: Tripoli is an abrasive material obtained from deposits of silicic acid. It is used for fine grinding of brass, aluminum, and other precious metals. Quartz, emery, and diamond are, however, natural abrasives.
5. A high-temperature electric arc of _____ is used for the manufacture of silicon carbide.
a) 1500oF
b) 3000oF
c) 4500oF
d) 7500oF
View Answer
Explanation: Silicon carbide is an artificially manufactured abrasive material. This is done by mixing sand, coke, and sawdust and passing it through an electric arc at 4500oF. This mixture is converted into silicon carbide and crushed.
6. Which abrasive has a common trade name as carborundum?
a) Aluminum oxide
b) Silicon carbide
c) Tungsten carbide
d) Boron carbide
View Answer
Explanation: Silicon carbide (SiC) is an artificial abrasive used for making grinding wheels, pipes, and pumps. It is better known by its trade name of carborundum. Aluminum oxide is another artificial abrasive.
7. Which of the following is regarded as a modern abrasive?
a) Tripoli
b) Silicon carbide
c) Pumica
d) Boron carbide
View Answer
Explanation: Boron carbide and tungsten carbide are generally regarded as modern abrasives. They are close to the hardness of a diamond, which is a natural abrasive. Boron carbide is used for polishing pastes.
8. The polishing of cast iron and finishing of stainless steels are applications of ______
a) Silicon carbide
b) Boron carbide
c) Tungsten carbide
d) Aluminum oxide
View Answer
Explanation: Aluminum oxide is an abrasive which is obtained by heating of aluminum salts or from ores of bauxite. It has a lighter color compared to silicon carbide and is not as hard, but is more resistant to impact. It is used for polishing cast irons, non-ferrous materials, and for giving a lustrous finish to stainless steels.
9. Which silicate structure does the following diagram represent?
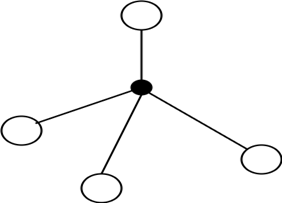
a) Tetrahedron
b) Poly-tetrahedral
c) Chain
d) Sheet
View Answer
Explanation: The given figure denotes the silicon-oxygen tetrahedron structure. It is the primary structural unit of silicates. In such a structure, one silicon atom is connected with four oxygen atoms.
10. What is the composition of a double tetrahedral structure?
a) SiO4
b) Si2O7
c) Si3O9
d) Si5O9
View Answer
Explanation: A double tetrahedral silicate structure is produced as a result of overcoming the deficiency of electrons in oxygen. Its composition is Si2O7 whereas that of a polyhedral unit is Si3O9.
11. Which of the following structures can be seen for the vitreous form of glass?
a) 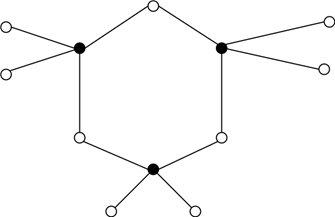
b) 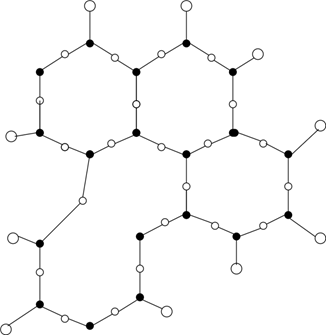
c) 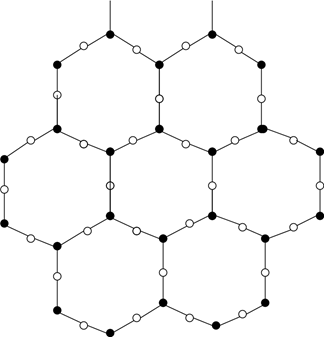
d) 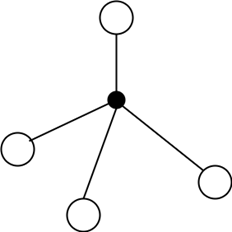
View Answer
Explanation: Glass is a vitreous silicate having a vitreous structure. It consists of a three-dimensional structure of covalent bonds. The figure represents the vitreous nature of silica in two-dimensional form.
12. Conversion of a tetrahedral unit into a three-dimensional structure gives a ______ structure.
a) Vitreous
b) Sheet
c) Chain
d) Framework
View Answer
Explanation: When a silicate tetrahedral unit extends into three dimensions, it gives a framework structure. On the other hand, a sheet structure extends into a two-dimensional plane similar to how framework extends in three-dimensions.
13. Quartz and Feldspar are examples having ______ structure
a) Tetrahedron
b) Sheet
c) Poly tetrahedral
d) Framework
View Answer
Explanation: When a silicate tetrahedral unit extends into three dimensions, it gives a framework structure. It has a relatively low density, low atomic packing factors, and good hardness. Common examples of framework structures can be found in cristobalite, quartz, and Feldspar.
14. Which silicate structure does the following figure represent?
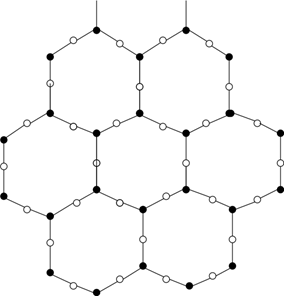
a) Chain
b) Sheet
c) Vitreous
d) Tetrahedron
View Answer
Explanation: A silicate sheet structure is found when a double chained structure extends into a two-dimensional plane. Clay and mica are examples of ceramics having a sheet structure.
15. The ability of a material to exist in more than one crystal structure is known as _______
a) Polymorphism
b) Allotropy
c) Polyhedral phase
d) Lattice
View Answer
Explanation: Polymorphism is the property of a material which allows it to exist in more than one form of space lattice or crystal structure. When such a process is reversible, it is defined as allotropy.
Sanfoundry Global Education & Learning Series – Engineering Materials & Metallurgy.
To practice all areas of Engineering Materials and Metallurgy, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Metallurgical Engineering MCQs
- Check Engineering Materials Books
- Check Metallurgical Engineering Books
- Apply for Metallurgical Engineering Internship
