This set of Mechanical Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Plastic Deformation of Single Crystal – Lattice Defects”.
1. Which of the following is Not a structure insensitive property?
a) Elastic modulus
b) Melting point
c) Coefficient of thermal expansion
d) Electrical resistivity
View Answer
Explanation: The Elastic modulus, melting point, and coefficient of thermal expansion are structure insensitive properties as it does not change with the internal change in the material such as defects. But electrical resistivity is structure sensitive property. For example, the resistivity of the material increases with an increase in dislocation density.
2. Which of the following is Not a structure sensitive property?
a) Yield strength
b) Fracture Strength
c) Semiconductor property
d) Specific heat
View Answer
Explanation: The Yield strength, fracture Strength, and semiconductor property depends upon the structure of the material. But specific heat is material defined property and does not change with internal defects of the material.
3. Which of the following is not a point defect?
a) Vacancy
b) Interstitial atom
c) Impurity atom
d) Dislocation
View Answer
Explanation: When the deviation from the periodic arrangement of the lattice is localized to the vicinity of only a few atoms or single atom, it is called a point defect, or point imperfection. The vacancy, interstitial atom, impurity atoms are only single lattice point defects. But dislocation is line defect.
4. Which of the following defect is a thermodynamically stable defect?
a) Vacancy
b) Interstitial atom
c) Dislocation
d) Stacking fault
View Answer
Explanation: A vacancy or vacant lattice site exists when an atom is missing from a normal lattice position. In pure metals, small numbers of vacancies are created by thermal excitation, and these are thermodynamically stable at temperatures higher than absolute zero. Rest of the defects are not the equilibrium defects.
5. For a given metal, the energy required to move an atom from the interior of a crystal to its surface is E=1eV (0.16*10-18). Find the fraction of the vacant lattice site in metal at 500 degree Celsius.
a) 3.06*10-7
b) 1.02*10-10
c) 6.27*10-3
d) 8.2*10-18
View Answer
Explanation: Fraction of the vacant lattice site is given as:
n/N=e-(E/kT)
Where n is the number of vacant sites in N sites, k is Boltzmann’s constant and temperature is in kelvin.
n/N = e(-0.16*10-18/(1.38*10-23 * (500+273)))
=> n/N = 3.06*10-7.
6. The dislocation is considered as the region of localized lattice disturbance separating the slipped and unslipped areas in a crystal.
a) True
b) False
View Answer
Explanation: The movement of dislocation is possible only when atoms in the plane is moved by one atomic distance. The region from dislocation has passed a region called slip region because all the atoms had already moved one atomic distance. And dislocation in advancing in the area where atoms are yet to move is the unslipped part.
7. In a positive edge dislocation, the atomic arrangement results in a ____________ above the slip plane and a ______________ below the slip plane.
a) compressive stress, tensile stress
b) tensile stress, compressive stress
c) compressive stress, compressive stress
d) tensile stress, tensile stress
View Answer
Explanation: In positive edge dislocation, there is an extra plane of atom above the slip plane. This causes a compression stress in this region, while below the slip plane one plane is missing, which result in tensile stress.
8. A pure edge dislocation can move in direction perpendicular to its length by _________ however, it may move vertically (along its length) by a process known as ______
a) glide, climb
b) climb, glide
c) kink, glide
d) kink, jog
View Answer
Explanation: An edge dislocation can move in a slip plane easily by glide or slip. If the edge dislocation wants to move in the plane perpendicular to slip plane, it is required to add or remove an atom which results in a climb of the dislocation. The movement by climb is diffusion controlled.
9. The burger vector is perpendicular to the dislocation line in ____________ while it is parallel for ________________
a) screw dislocation, edge dislocation
b) edge dislocation, screw dislocation
c) edge dislocation, mixed dislocation
d) mixed dislocation, screw dislocation
View Answer
Explanation: The burger vector is perpendicular to the dislocation line in edge dislocation, while it is parallel for screw dislocation.
Following diagram shows the edge dislocation structure
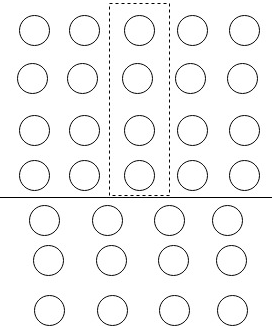
Dislocation and burger vector are perpendicular to each other. In the case of screw dislocation, dislocation line and burger vector are parallel to each other.
Sanfoundry Global Education & Learning Series – Mechanical Metallurgy.
To practice all areas of Mechanical Metallurgy, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Metallurgical Engineering MCQs
- Check Metallurgical Engineering Books
- Check Mechanical Metallurgy Books
- Apply for Metallurgical Engineering Internship
