This set of Mechanical Metallurgy Interview Questions and Answers for freshers focuses on “Plastic Deformation – Concept of Crystal Geometry – 2”.
1. The Miller indices of the given plane in the hexagonal closed packed system are equal to ________
Given that a1, a2, a3, are three base axes, and b1 is the vertical axis of the HCP structure.

a) \((01\bar{1}1)\)
b) \((0\bar{1}\bar{11})\)
c) (1000)
d) (1100)
View Answer
Explanation: The miller indices of the plane are obtained by finding the intersection of the plane of all the four crystallographic directions.
The plane is parallel to the a1 axis, cuts a2 at 1, intercepts a3 in negative one direction, and intercepts along the b direction at 1. So, the Miller indices for the plane is \((01\bar{1}1)\).
2. Find the effective number of atoms in Body-centered cubic lattice?
a) 1
b) 2
c) 3
d) 4
View Answer
Explanation: In the BCC structure, there are eight atoms at every corner which contributes 1/8 part.
=> 1/8*8=1
Also, there is an atom in the center of the cube contributing to 1 atom.
So, the total number of effective atoms 1+1=2.
3. Find the effective number of atoms in Face-centered cubic lattice?
a) 1
b) 2
c) 3
d) 4
View Answer
Explanation: In FCC structure, there are six atoms on every face which contributes ½ atom. Also, at every corner, there are atoms which contributes 1/8.
=> 1/2*6+1/8*8= 3+1=4 atoms.
4. Stacking sequence for Hexagonal closed packed structure is ____________
a) ABABABAB………
b) ABCABCABC………
c) AAAAAAAA
d) ABABCABABC
View Answer
Explanation: The stacking sequence represents the way of arrangement of atomic layers over one another. HCP is a closely packed structure, where atom in a layer is surrounded by six atoms touching in the plane. In the stacking process, the next layer sits in the valley. Will occupy a valley which is just above the first atomic layer, so ABABABABAB pattern.
5. Stacking sequence for Face-centered cubic structure is ____________
a) ABABABAB………
b) ABCABCABC………
c) AAAAAAAA
d) ABABCABABC
View Answer
Explanation: The stacking sequence represents the way of arrangement of atomic layers over one another. FCC is a closely packed structure, where atom in a layer is surrounded by six atoms touching in the plane. In the stacking process, the next layer sits in the valley. Will occupy a valley which is just above the first atomic layer so ABCABCABCABCABC pattern.
6. For hexagonal closed packed structure ideal c/a ratio is equal to _____
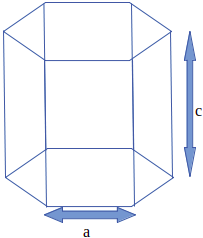
a) 1
b) 2
c) 1.633
d) 1.33
View Answer
Explanation: The ideal HCP structure has closed packed structure with c/a ratio being:
\(\sqrt{\frac{8}{3}}=\sqrt{2.6666}\)
=> 1.633.
7. The atomic packing efficiency of FCC and Simple cubic is __________
a) 74% and 52%
b) 74% and 68%
c) 68% and 52%
d) 68% and 74%
View Answer
Explanation: The atomic packing efficiency or packing factor for FCC and HCP is 74%. For BCC it is equal to 68%. The simple cubic structure has 52% packing efficiency and diamond cubic it is just 34%.
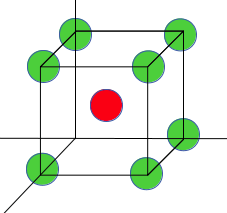
8. Find the atomic density (atoms per unit area) for (111) plane of BCC lattice?
Given that the lattice parameter is a.
a) 1/√3a2
b) 4/√3a2
c) 2/√3a2
d) √3/a2
View Answer
Explanation: atomic density=(Number of atoms in the plane)/(area of the plane)
BCC Unit cell is given here:
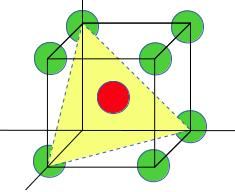
The (111) plane in BCC lattice:
So, the effective number of atoms in (111) plane:
(1) every corner atom is contributing 1/6 atoms, so total three atoms = 1/6*3 = 1/2 atoms
(2) The central atom does not pass through the (111) plane (be careful with this, a lot of students make the mistake of taking central atom in the calculation).
So total atoms will 1/2=3/2.
The total area of the (111) plane is;
Side of the plane √2a, where a is the side of cube.
Area of equilateral triangle = √3/4 (side)2.
So, the total area = √3/4 (√2a)2 = √3/2a2.
atomic density = (1/2)/(√3/2a2).
=> 1/√3a2.
9. Find the atomic density (atoms per unit area) for (110) plane of FCC lattice? (Given that the lattice parameter is a).
a) 2/√2a2
b) 1/√2a2
c) 2/√3a2
d) 3/√3a2
View Answer
Explanation: atomic density=(Number of atoms in the plane)/(area of the plane)
FCC Unit cell is given here:
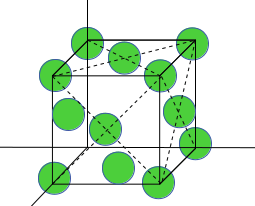
The (110) plane is shown here:
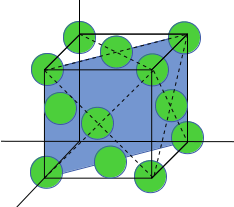
Total number of atoms in (110) plane=4 corner atom (each contribute ¼) + 2 face center atom (each contribute ½).
Total area of plane= a* √2a= √2a2
atomic density=2/√2a2.
10. The plastic deformation is generally confined to the low-index planes, which have a higher density of atoms per unit area than the high-index planes.
a) True
b) False
View Answer
Explanation: 2 things play a role in deciding the deformation of the plastic:
(1) distance between atoms in the same planes (it should be low for easy plastic deformation).
(2) distance between 2 planes of the same miller indices (it should be high for easy plastic deformation).
So, the plastic deformation is generally confined to the low-index planes, which have a higher density of atoms per unit area.
Sanfoundry Global Education & Learning Series – Mechanical Metallurgy.
To practice all areas of Mechanical Metallurgy for Interviews, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Metallurgical Engineering MCQs
- Check Mechanical Metallurgy Books
- Apply for Metallurgical Engineering Internship
- Check Metallurgical Engineering Books
