This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Adsorption”.
1. Which of the following is not an adsorbent?
a) Carbon
b) Polymers and resins
c) Clay
d) Dry sponge
View Answer
Explanation: A sponge will absorb or take in water from another area and put it inside of itself. A dry sponge can hold more water than a wet sponge is closer to saturation and as such cannot hold more water. Sponges with more tiny holes can absorb more water than the sponges with less tiny holes and thus leads to the absorption process.
2. What do you mean by the term “Sorption”?
a) Attachment
b) Detachment
c) Diffusion
d) Thermal Expansion
View Answer
Explanation: Sorption is a physical and chemical process by which one substance becomes attached to another. The reverse of sorption is desorption.
3. The desorption curve is higher than the adsorption curve.
a) True
b) False
View Answer
Explanation: Theoretically, desorption curve is higher than adsorption curve in low pressure area if the material is mesoporous (2-50nm). If the material is microporous (<2nm), both curves should be matched together.
4. Which of the following isotherm is applicable to physical adsorption?
a) Langmuir
b) BET
c) Freundlich
d) Kisluik
View Answer
Explanation: The Freundlich isotherm was the first isotherm model proposed for sorption processes. It can be applied for non ideal sorption on heterogeneous surfaces, as well as, multilayer sorption. A variation in the slope between 0 and 1 is associated with a chemisorption process, which is more heterogeneous as the value gets closer to 0. Due to the lack in fundamental thermodynamic basis, since there is no approach to Henry’s law at vanishing concentrations, this represents a limitation of this isotherm model.
5. Which type of isotherm is given from the figure, Choose from the following options?
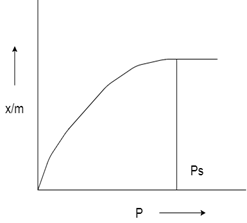
a) Type 1 Adsorption isotherm
b) Type 2 Adsorption isotherm
c) Type 3 Adsorption isotherm
d) Type 4 Adsorption isotherm
View Answer
Explanation: The above graph depicts Monolayer adsorption. This graph can be easily explained using Langmuir Adsorption Isotherm. Examples of Type-I adsorption are Adsorption of Nitrogen (N2) or Hydrogen (H) on charcoal at temperature near to -1800C.
6. Calculate the adsorption of a dye on activated carbon at 25°C, where k = 0.025, n = 0.5 and C = 0.04.
Based on the Freundlich isotherm.
a) 0.050
b) 0.030
c) 0.040
d) 0.060
View Answer
Explanation: Given data
n = 0.5
Kd = 0.025
C = 0.04
Substitute the values in the corresponding equation
q = Kd C(1/n)
q = (0.025) (0.04)(1/0.5)
q = 0.040.
7. Which of the following statements regarding the physical adsorption of a gas on surface of solid is not correct?
a) On increasing temperature, adsorption increases continuously
b) Enthalpy changes are negative
c) Adsorption is specific
d) It is reversible in nature
View Answer
Explanation: Physisorption is exothermic in nature. Therefore according to le chateliars principle, it occurs readily at low temperature and decreases with increase in temperature. Bond between adsorbent i.e. surface and adsorbate like gases are weak so when temperature is increasing the bond get break easily and adsorption of adsorbate get stop so rate will decrease on increasing temperature.
8. Which of the following is not characteristic of chemisorption?
a) It is irreversible
b) It is specific
c) It is multilayer phenomenon
d) Heat of adsorption is about 400kj
View Answer
Explanation: Chemisorption involves formation of chemical bonds between adsorbate and adsorbent molecules. Once the valency is satisfied, the adsorbent molecules can’t form bond with more adsorbate molecules. Thus only one layer is formed.
9. For an adsorbant-adsorbate system obeying the Langmuir adsorption isotherm, b = 0.48 bar-1 and p = 0.16 bar-1. At what pressure will 50% of the surface be covered?
a) 0.05 bar
b) 0.07 bar
c) 0.08 bar
d) 0.04 bar
View Answer
Explanation: Given data
b = 0.48 bar-1
p = 0.16 bar-1
Substitute in the corresponding equation
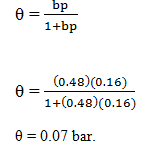
10. Adsorption of methane follows the Langmuir adsorption isotherm at 90K. If p = 1.896cm3g-1bar-1 and b = 0.146bar-1. Calculate the value of θ.
a) 0.116 bar
b) 0.514 bar
c) 0.214 bar
d) 0.216 bar
View Answer
Explanation: Given data
p = 1.896cm3g-1 bar-1
b = 0.146 bar-1
Substitute in the corresponding equation
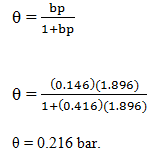
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Biotechnology Books
- Apply for Biotechnology Internship
- Practice Biotechnology MCQs
- Check Bioprocess Engineering Books
