This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Measurement of KLa”.
1. What do you mean by “kLa”?
a) Volumetric mass transfer coefficient
b) Henry’s law coefficient
c) Volumetric oxygen transfer coefficient
d) Volumetric Solute transfer coefficient
View Answer
Explanation: The value of the specific exchange surface (a) is difficult to determine for small bubbles found in a bioreactor. So, the entire term “kLa” is often called the volumetric oxygen transfer coefficient.
2. For which type of mass transfer does Oxygen-Balance method is used?
a) Gas- Gas
b) Gas-Solid
c) Gas-liquid
d) Liquid-Liquid
View Answer
Explanation: This technique is based on the equation for gas-liquid mass transfer. The oxygen content of gas streams flowing to and from the fermenter is measured.
3. What is the proper concentration unit of kLa?
a) h-1
b) ml/h
c) mmol/h
d) ml/sec
View Answer
Explanation: In this relation, the volumetric oxygen transfer coefficient, kLa, has the units of mmol, of O2/ml. h. unit concentration gradient. Using the proper concentration units, kLa has the unit of reciprocal of time (i.e., time-1).
4. Which of the following is the efficient value for oxygen concentration?
a) Equal to Ccrit
b) Below Ccrit
c) Above Ccrit
d) Ccrit
View Answer
Explanation: It is important that the oxygen concentration remains above Ccrit so that the rate of oxygen uptake by the cells is independent of oxygen level.
5. A 20-1 stirred fermenter containing a Bacillus thuringiensis culture at 30°C is used for production of microbial insecticide, kLa is determined using the dynamic method. Air flow is shut off for a few minutes and the dissolved-oxygen level drops; the air supply is then re-connected. When steady state is established, the dissolved-oxygen tension is 78% air saturation. The following results are obtained. Estimate kLa.
| Time(s) | 5 | 15 |
|---|---|---|
| Oxygen tension (% air saturation) | 50 | 66 |
a) 0.080 s-1
b) 0.083 s-1
c) 0.085 s-1
d) 0.081 s-1
View Answer
Explanation: \(\bar{(C_{AL})}\) = 78% air saturation. Let us define t1 = 5s, CAL1 = 50%, t2 = 15 s and CAL2 = 66%. From the equation:
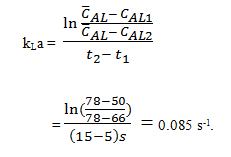
6. Refer to Q5 and, calculate: An error is made determining the steady-state oxygen level which, instead of 78%, is taken as 70%. What is the percentage error in kLa resulting from this 10% error in \(\bar{C_{AL}}\)?
a) 50%
b) 25%
c) 75%
d) 100%
View Answer
Explanation: If \(\bar{C_{AL}}\) is taken to be 70% air saturation:
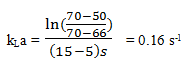
The error in kLa is almost 100%.
7. “The kLa value will depend upon the design and operating conditions of the fermenter”, is this statement true or false?
a) True
b) False
View Answer
Explanation: The value of kLa is unique to both the size and configuration of a reactor vessel. Although some empirically derived expressions have been published for predicting kLa values in non-Newtonian fluids, there is no agreed upon set of equations that account for all of the variables that can affect the results. Accordingly, predicting kLa is not possible, and kLa studies need to be performed for bioreactors individually. The kLa value will depend upon the design and operating conditions of the fermenter and will be affected by the variables such as
– aeration rate,
– agitation rate and
– impeller design.
8. Which of the following technique does not require the measurement of dissolved oxygen concentrations?
a) Dynamic gassing out method
b) Static gassing out method
c) Oxygen-Balance method
d) Sulphite oxidation method
View Answer
Explanation: The oxygen-transfer rate is determined by the oxidation of sodium sulphite solution. This technique does not require the measurement of dissolved oxygen concentrations.
As oxygen enters solution it is immediately consumed in the oxidation of sulphite, so that the sulphite oxidation rate is equivalent to the oxygen-transfer rate. Since the dissolved oxygen concentration, is zero then the kLa may then be calculated from the equation:
kLa = OTR / C*
where OTR is the oxygen transfer rate.
9. Speed is the factor affecting the value of kLa.
a) True
b) False
View Answer
Explanation: Because kLa measurements involve monitoring levels of DO following a system perturbation, the results can be influenced by the response time (or “speed”) of a sensor making those determinations. Sensors with response times (τr) on the order of the first-order time constant of the mass transfer (1 / kLa) require special treatment of their data to correct for the time lag in readings introduced by the oxygen sensor.
10. Which of the following is not a chemical method to measure kLa?
a) Dynamic gassing out method
b) Sodium sulfite oxidation method
c) Carbon dioxide absorption method
d) Glucose oxidase method
View Answer
Explanation: This method hinges on the measurement of the dissolved oxygen concentration that is altered by absorption or desorption, facilitated by flushing with inert gases like nitrogen. The instantaneous dissolved oxygen concentration can be measured by using electrodes and kLa is hence estimated from the slope of the resulting plot.
11. Which of the following does not affect KLa value?
a) Air flow rate
b) Presence of enzymes
c) Presence of antifoam agents
d) Degree of agitation
View Answer
Explanation: The mass transfer coefficient is strongly affected by agitation speed and air flow rate. The mass transfer coefficient increases with agitation speed and air flow rate. Since fermentation is usually conducted at constant temperature and pressure so thermodynamically antifoam agents make the foam unstable causing ΔG < va ΔA (where ΔG is the free energy change, va is the surface tension and ΔA is the change in area).
12. A 10,000 liter (of liquid) bioreactor contains 5 g / L of growing cells qO2 = 20 mmoles O2 / (g cells hr) DT = 2 m, DI = 1 m, (6 – blade turbine agitator) x 3 blades and CL = 1 mg O2/L. Calculate OUR.
a) 200 mmoles O2 / (g cells hr)
b) 250 mmoles O2 / (g cells hr)
c) 100 mmoles O2 / (g cells hr)
d) 150 mmoles O2 / (g cells hr)
View Answer
Explanation: OUR = X qO2 = (5 g / L) (20 mmoles O2 / (g cells hr)) = 100 moles O2 / (g cells hr).
13. Refer to Q12 and, calculate OTR. (Given: kLa = 169 mmol O2/ 1 hr atm, P* = 0.0263 atm and PO2 = 0.21 atm).
a) 30.05 mmoles O2 / liter hr
b) 31.05 mmoles O2 / liter hr
c) 20.05 mmoles O2 / liter hr
d) 21.05 mmoles O2 / liter hr
View Answer
Explanation: OTR = kLa(PO2 – P*)
= 169 mmol O2 / 1 hr atm(0.21- 0.0263) atm
= 31.05 mmoles O2 / liter hr.
14. From Q12 and Q13, which of the following condition is relevant?
a) OTR>OUR
b) OTR<OUR
c) OTR=OUR
d) OTR≠OUR
View Answer
Explanation: Since OUR > OTR, we must modify the bioreactor operation in order to bring them into balance:
• increase N
• use pure O2 rather than air.
15. KLa is measured in the absence of microorganisms?
a) True
b) False
View Answer
Explanation: The methods to measuring the kLa in a microbial bioprocess can be classified into the absence of microorganisms or with dead cells and in the presence of biomass that consumes oxygen at the time of measurement.
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Biotechnology Internship
- Check Biotechnology Books
- Practice Biotechnology MCQs
- Check Bioprocess Engineering Books
