This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Enthalpy Change in Non-Reactive Processes”.
1. Heat transferred to raise or lower the temperature of a material is called Specific heat.
a) True
b) False
View Answer
Explanation: Heat transferred to raise or lower the temperature of a material is called sensible heat; change in the enthalpy of a system due to variation in temperature is called sensible heat change, whereas the term specific heat refers to heat capacity expressed on a per-unit-mass basis.
2. The symbol “Û” refers to?
a) Amount
b) Rate of enthalpy
c) Molar flow rate
d) Specific internal energy
View Answer
Explanation: The symbol “Û” refers to Specific internal energy in a close system in Enthalpy change in non-reactive processes whereas in an open- system symbol “Ĥ” refers to specific enthalpy.
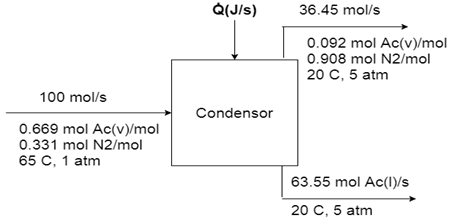
3. Acetone (denoted as Ac) is partially condensed out of a gas stream containing 66.9 mole % acetone vapor and the balance nitrogen. Process specifications and material balance calculations lead to the flowchart shown below.
The process operates at steady state. Calculate the required cooling rate.
Reference states for acetone and nitrogen are-
N2 (g, 25°C, 1 atm), Ac (l, 20°C, 5 atm)
a) – 2390kW
b) – 2320kW
c) – 3560kW
d) – 3570kW
View Answer
Explanation: Basis: 100 mole/s of mixed gas (Ac & N2)
Write and simplify the energy balance.
Note the following points about the table –
(i) Nitrogen has only one state for inlet and outlet.
(ii) Acetone has one inlet state but two outlet state
(iii) Since the liquid acetone leaving the system is at the reference state, so its specific enthalpy is zero.
Calculate all unknown specific enthalpies –

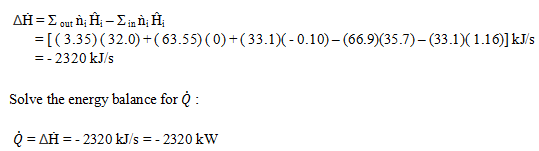
4. Calculate how much energy (kJ) is required to heat a bottle containing 300 mL of liquid water from room temperature (20 °C) to its normal boiling point (100 °C), while still remaining a liquid.
a) 104.4 kJ
b) 100.4 kJ
c) 140.4 kJ
d) 104.5 kJ
View Answer
Explanation: Enthalpy (“Water”, 20, 000,”l”)
= 6.032 kJ/mol \((∫_{20}^{100} C_{pH_2O} dT)\)
= \( (\frac{6.032 kJ}{mol}) (\frac{1g}{ml}) (\frac{mol}{18.02 g}) (\frac{300 ml}{1})\) = 100.4 kJ
5. Calculate ΔH for a process in which 2.0 mole of NaOH is dissolved in 400 mol H2O at 25C.
(\(\Delta \hat{H}_{s}\) at r = 200, 25°C is – 42.26 kJ/ mol)
a) –84.52 kJ
b) -80.42 kJ
c) –64.52 kJ
d) –60. 42 kJ
View Answer
Explanation: r = 400/2 = 200 mol H2O/ mol NaOH
ΔH = n \(\hat{H}_{s}\) = 2 (- 42.26) = – 84.52 kJ
6. What term is used for Temperature measured by Thermometer?
a) Wet- bulb temperature
b) Dry- bulb temperature
c) Dew point temperature
d) Normal temperature
View Answer
Explanation: The dry-bulb temperature (DBT) is the temperature of air measured by a thermometer freely exposed to the air but shielded from radiation and moisture. DBT is the temperature that is usually thought of as air temperature, and it is the true thermodynamic temperature.
7. Estimate the following properties of humid air at 41°C and 10% relative humidity:
Absolute humidity = 0.0048 kg H2O/ kg DA
Wet-bulb temperature = 19°C
Humid volume = 0.895 M3/ kg DA
Dew point = 3°C
Specific enthalpy = 54.2 – 0.7 = 53.5 kJ/ kg DA
What is the amount of water in 150 m3 of air at these conditions?
a) 0.605 kg H2O
b) 0.705 kg H2O
c) 0.805 kg H2O
d) 0.905 kg H2O
View Answer
Explanation: \((\frac{150 m^3\,humid \,air}{1}) (\frac{kg \,DA}{0.895 \,M^3}) (\frac{0.0048 \,kg \,H_2O}{kg \,DA})\) = 0.805 kg H2O
8. What is the enthalpy of 130 g formic acid at 70°C and 1 atm relative to 25°C and 1 atm?
Cp for formic acid in the temperature range of interest is 0.524 cal g-1 °C-1.
a) 4.68 kcal
b) 3.06 kcal
c) 2.06 kcal
d) 2.68 kcal
View Answer
Explanation: ΔH = ( 130 g) (0.524 cal g-1 °C-1) (70-25)°C
ΔH = 3065.4 cal or
ΔH = 3.06 kcal
Relative to H=0 at 25°C the enthalpy of formic acid at 70°C is 3.06 kcal.
9. Processes for phase change of vapour to liquid is called?
a) Vaporization
b) Fusion
c) Sublimation
d) Condensation
View Answer
Explanation: Condensation is the change of the physical state of matter from gas phase into liquid phase, and is the reverse of evaporation.
10. 50 g benzaldehyde vapour is condensed at 179°C, What is the enthalpy of the liquid relative to the vapour?
Given: The molecular weight of benzaldehyde is 106.12, the normal boiling point is 179.0°C and the standard heat of vaporization is 38.40 kJ gmol-1. For condensation the latent heat is – 38.40 kJ gmol-1.
a) -18.09 kJ
b) -17.09 kJ
c) – 18.06 kJ
d) – 17.06 kJ
View Answer
Explanation: ΔH = 50 g (- 38.40 kJ gmol-1). |(1 gmol)/(106.12 g)| = -18.09 kJ
Therefore, the enthalpy of 50 g benzaldehyde liquid relative to the vapour at 179°C is – 18.09 kJ. As heat is released during condensation, the enthalpy of the liquid is lower than the vapour.
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Bioprocess Engineering Books
- Check Biotechnology Books
- Practice Biotechnology MCQs
- Apply for Biotechnology Internship
