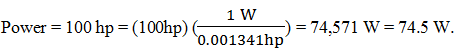This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Basic Energy Concepts”.
1. High energy input for downstream processing is maximized.
a) True
b) False
View Answer
Explanation: Energy input for downstream processing is minimised to avoid damaging heat-labile products. Nevertheless, energy effects are important because biological catalysts are very sensitive to heat and changes in temperature. In large-scale processes, heat released during reaction can cause cell death or denaturation of enzymes if it is not quickly removed.
2. Which of the following is not an intensive property?
a) Temperature
b) Density
c) Mass
d) Mole fraction
View Answer
Explanation: Temperature, density, and mole fraction are examples of properties which are independent of the size of the system; these quantities are called intensive variables. On the other hand, mass, volume and energy are extensive variables which change if mass is added to or removed from the system.
3. How much heat is produced by a human body?
a) 200 W
b) 100 W
c) 50 W
d) 0.5 W
View Answer
Explanation: A man doing no or very little physical work needs about 2,000 kcal (or less) of energy in his daily food. The body converts this energy almost entirely into heat.
1 day = 24 x 60 x 60 s = 86,400 s 1 cal = 4.2 J
Hence, 2000 kcal/day = 2000 × 4.2 kj/day = \(\frac{8.4 \,Mj}{86600s}\)
= 100 j/s = 100 W
We see that a human body doing no work is equivalent to a heat source of about 100 W – the equivalent of a good bulb.
4. 1 watt is equal to how much horsepower (hp) unit?
a) 0.001341 hp
b) 0.001241 hp
c) 0.001141 hp
d) 0.001151 hp
View Answer
Explanation: 1 watt = 1 W = 1 J/1 s = 10-3 kW = 10-6 MW
= 3.412 BTU/h
= 0.001341 hp (horsepower units).
5. An automobile has a horsepower rating (power) of 100 hp. Calculate its power in watts.
a) 75.5 kW
b) 74.5 kW
c) 75.4 kW
d) 74.4 kW
View Answer
6. Given the following information about a system, calculate specific enthalpy (in Btu/lbm).
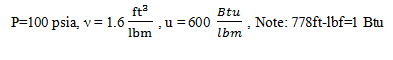
a) 629.6 btu/lbm
b) 639.6 btu/lbm
c) 660.9 btu/lbm
d) 640.9 btu/lbm
View Answer
Explanation: h = u + Pv
h = \(600 \frac{Btu}{lbm} + (100 \frac{lbf}{in^2}) (1.6 \frac{ft^3}{lbm}) (144 \frac{in^2}{ft^2}) (\frac{Btu}{778 \,ft-lbf})\) = 629.6 btu/lbm.
7. At what rate per hour does a 1 kW heater convert electrical energy into heat?
a) 3.5 MJ
b) 2.5 MJ
c) 3.6 MJ
d) 2.6 MJ
View Answer
Explanation: Energy = power x time
1 kW is 1,000 watts
Since 1 watt is 1 joule per sec
1,000 watts is 1,000 joules per sec
In one hour there are 3,600 seconds
Substituting in the equation energy = 1,000 x 3,600 joules
= 3,600,000 joules
= 3.6 MJ
Therefore a 1 kW heater converts 3.6 MJ of electrical energy into heat per hour.
8. Water flows between two points 1, 2. The volumetric flow rate is 20 litres/min. Point 2 is 50 m higher than point 1. The pipe internal diameters are 0.5 cm at point 1 and 1 cm at point 2. The pressure at point 2 is 1 atm. Calculate the pressure at point 1.
a) 4.6 bar
b) 5.6 bar
c) 4.1 bar
d) 5.1 bar
View Answer
Explanation: ΔP/ρ + Δv2/2 + gΔh + F = W
ΔP = P2 – P1 (Pa)
Δv2 = v22 – v12
Δh = h2 – h1 (m)
F = frictional energy loss (mechanical energy loss to system) (J/kg)
W = work done on system by pump (J/kg)
ρ = 1000 kg/m3
Volumetric flow is 20/ (1000.60) m3/s
= 0.000333 m3/s
v1 = 0.000333/(π(0.0025)2) = 16.97 m/s
v2 = 0.000333/ (π(0.005)2) = 4.24 m/s
(101325 – P1)/1000 + [(4.24)2 – (16.97)2]/2 + 9.81.50 = 0
P1 = 456825 Pa (4.6 bar).
9. Determine the density, specific gravity, and mass of the air in a room whose dimensions are 4m × 5m × 6m at 100 kPa and 25° C.
a) 1.16 kg/m3, 0.00116, 140 kg
b) 1.17 kg/m3, 0.00117, 140 kg
c) 1.15 kg/m3, 0.00115, 140 kg
d) 1.14 kg/m3, 0.00114, 140 kg
View Answer
Explanation: At specified conditions, air can be treated as ideal gas.
The gas constant of air is R = 0.287 k Pa-m3/Kg K
The density of the air is determined from the ideal-gas relation, P = ρ R T to be
ρ = P/RT = (100 kPa)/((0.287 k Pa-m3/Kg K) (25+273.15)K) = 1.17 kg/ m3
Then the specific gravity of the air becomes
SG = (ρ)/ρH2O = (1.17 kg/m3)/(1000 kg/m3) = 0.00117
Finally, the volume and mass of the air in the room are
V = (4m) (5m) (6m) = 120 m3
m = ρV = (1.17 kg/m3) (120 m3) = 140 kg.
10. Extensive properties are linearly dependent on the amount of substance.
a) True
b) False
View Answer
Explanation: Extensive properties are linearly dependent on the amount of substance. Examples: mass, volume, energy. Take two identical samples with all properties identical and combine them into a single sample. Properties that double are extensive. Properties that remain the same are intensive. For example, if I took 1.0 liter of water at room temperature (25 °C) and added another 1.0 liter of water at the same temperature then I would have 2.0 liters of water at 25 C. From this example we see that Volume and Mass are extensive properties (i.e., volume and mass doubled), while Temperature is an intensive property (i.e., temperature stayed the same). You would also expect the density to remain the same, so it is also an intensive property.
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Biotechnology Internship
- Check Biotechnology Books
- Check Bioprocess Engineering Books
- Practice Biotechnology MCQs