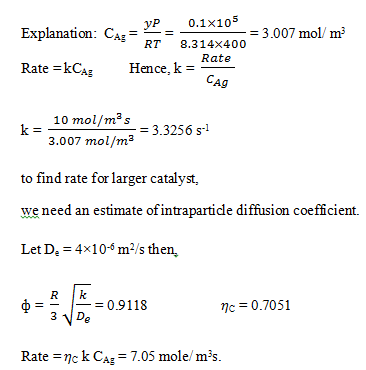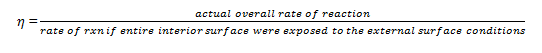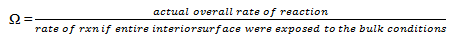This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Minimising Mass Transfer Effects”.
1. Thiele modulus is used for the solid catalyst.
a) True
b) False
View Answer
Explanation: The Thiele Modulus was developed to describe the relationship between diffusion and reaction rate in porous catalyst pellets with no mass transfer limitations. This value is generally used in determining the effectiveness factor for catalyst pellets.
2. Reducing the reaction rate rA,obs improves the effectiveness of mass transfer aimed at increasing the reaction rate.
a) True
b) False
View Answer
Explanation: When the catalyst is very active with a high demand for substrate, mass transfer is likely to be slow relative to reaction so that steep concentration gradients are produced. However, limiting the reaction rate by operating at sub-optimum conditions or using an organism or enzyme with low intrinsic activity does not achieve the overall goal of higher conversion rates. Because rA,obs is the reaction rate per volume of catalyst, another way of reducing rA,obs is to reduce the cell or enzyme loading in the solid. This reduces the demand for substrate per particle so that mass transfer has a better chance of supplying it at a sufficient rate. Therefore, if the same mass of cells or enzyme is distributed between more particles, the rate of conversion will increase.
3. Thiele modulus will increase with the decrease of the size of the catalyst.
a) True
b) False
View Answer
Explanation: Thiele modulus (Φ) is proportional to the square of catalyst size (R2 for spheres or b2 for flat plates), reducing the catalyst size has a more dramatic effect on Φ than changes in any other variable. It is therefore a good way to improve the reaction rate. In principle, mass-transfer limitations can be completely overcome if the particle size is decreased sufficiently.
4. “Increasing the bulk concentration of substrate CAb”, is included in which parameter?
a) Internal mass-transfer
b) External mass-transfer
c) Internal-External mass- transfer
d) Heat transfer
View Answer
Explanation: External mass transfer is more rapid at high bulk substrate concentrations; the higher the concentration, the greater is the driving force for mass transfer across the boundary layer.
5. The boundary layer is necessary for the analysis of data.
a) True
b) False
View Answer
Explanation: In large-scale reactors, external mass-transfer problems may be unavoidable if sufficiently high liquid velocities cannot be achieved. However, when evaluating biocatalyst kinetics in the laboratory, it is advisable to eliminate fluid boundary layers to simplify analysis of the data. Several laboratory reactor configurations allow almost complete elimination of interparticle and interphase concentration gradients. Operation with high liquid velocity through the bed reduces boundary-layer effects.
6. Increase in pump speed does not change the overall reaction rate.
a) True
b) False
View Answer
Explanation: Increase in pump speed do not change the overall reaction rate. Therefore, if we can identify a liquid velocity u*L at which reaction rate becomes independent of liquid velocity, operation at uL > u*L will ensure that ηC = 1.
7. Accessibility of the surface area of the catalyst is typically non-limiting.
a) True
b) False
View Answer
Explanation: Rate of catalytic surface reactions is proportional to the catalyst surface area. However, accessibility of that surface area is typically limiting, as most of it is inner surface area inside the porous catalyst structure. Essentially all the surface area of most typical technical catalysts is internal surface area. Hence, a catalyst can only be used at its maximum potential, if diffusion inside the pore structure is not limiting.
8. Rate of reaction over a finely crushed catalyst of radius of 0.5 mm was measured as 10.0 mole/sm3 catalyst. Temperature is 400 K and pressure is 105 Pa and mole fraction of reactant in the gas is 0.1. Find the rate for a catalyst of pellet radius of 3mm. (Assume ηC = 1 for small catalyst).
a) 7.01 mole / m3s
b) 7.03 mole / m3s
c) 7.05 mole / m3s
d) 7.07 mole / m3s
View Answer
9. The internal effectiveness factor is symbolized by ________
a) μ
b) Φ
c) η
d) Ω
View Answer
10. The overall effectiveness factor is symbolized by __________
a) μ
b) Φ
c) η
d) Ω
View Answer
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Biotechnology Internship
- Check Biotechnology Books
- Practice Biotechnology MCQs
- Check Bioprocess Engineering Books