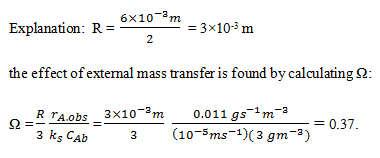This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “External Mass Transfer”.
1. When the boundary layer is present in the shell mass transfer then?
a) CAs = CAb
b) CAs ≠ CAb
c) CAs < CAb
d) CAs > CAb
View Answer
Explanation: The term CAs is the concentration of substrate A at the external surface of the catalyst. This term made its way into the analysis in the boundary conditions used for solution of the shell mass balance.
Reduction in substrate concentration from CAb in the bulk liquid to CAs at the catalyst surface occurs across the boundary layer surrounding the solid. In the absence of the boundary layer, CAS = CAb, which is easily measured. When the boundary layer is present, CAs takes some value less than CAb.
2. What is the dimension of liquid – phase mass-transfer coefficient “kS”?
a) LT-1
b) LT
c) L-1T-1
d) L-1T
View Answer
Explanation: Rate of mass transfer across a liquid boundary layer is represented by the following equation:
NA = kSa (CAb – CAS)
where NA is the rate of mass transfer, ks is the liquid-phase mass-transfer coefficient with dimensions LT-1, and a is the external surface area of the catalyst. If NA is expressed per volume of catalyst with units of, for example, kg mol s-1 m-3, to be consistent, a must also be expressed on a catalyst-volume basis with units of, for example, m2 m-3 or m-1.
3. If Ω << 1, then _________
a) CAS = CAb
b) CAS < CAb
c) CAS > CAb
d) CAS = CAb = 0
View Answer
Explanation: Ω is an observable modulus for external mass transfer. If Ω << 1, CAS = CAb and external mass-transfer effects are insignificant. Otherwise, CAS < CAb and external mass-transfer effects are significant.
4. Removing the boundary layer, CAS would decrease.
a) True
b) False
View Answer
Explanation: Removing the boundary layer would increase the value of CAS, thus establishing a greater driving force for internal mass-transfer and reducing the likelihood of CA falling to zero inside the particle.
5. Mass transfer resistance leads to an increase in the concentration of reactants at the catalyst surface in bulk fluid.
a) True
b) False
View Answer
Explanation: In absence of mass transfer there is no difference in concentration of reactant at bulk and near the catalyst surface. However, presence of significant mass transfer resistance results in a decrease in the concentration of reactants at the catalyst surface compared to that in the bulk fluid. Consequently, the observed rate is less than the intrinsic rate evaluated at bulk fluid reactant concentration.
6. Denitrifying bacteria are immobilised in gel beads and used in a stirred reactor for removal of nitrate from groundwater. At a nitrate concentration of 3 g m-3, the conversion rate is 0.011 g s-1 m-3 catalyst. The effective diffusivity of nitrate in the gel is 1.5×10-9 m2 s-1, the beads are 6 mm in diameter, and the liquid-solid mass-transfer coefficient is 10-5 m s-1. Km for the immobilised bacteria is approximately 25 g m-3. What is the value of an observable modulus for external mass transfer, Ω?
a) 0.35
b) 0.36
c) 0.37
d) 0.38
View Answer
7. Refer to Q6 and calculate the value of CAs.
a) 1.9 g m-3
b) 0.9 g m-3
c) 2.9 g m-3
d) 3.9 g m-3
View Answer
Explanation: CAs/CAb = 1 – Ω = 0.63
CAs = 0.63 CAb = 0.63 (3 gm-3) = 1.9 g m-3.
8. If the reaction is endothermic, the temperature at the catalyst surface will be less.
a) True
b) False
View Answer
Explanation: If the reaction is endothermic, the temperature at the catalyst surface will be less than the bulk fluid temperature and the observed rate will be less than that determined at the bulk fluid temperature.
9. Rate at the surface will be higher due to heat transfer limitations.
a) True
b) False
View Answer
Explanation: If the reaction is endothermic, the temperature at the catalyst surface will be less than the bulk fluid temperature and the observed rate will be less than that determined at the bulk fluid temperature. If the reaction is exothermic, the temperature of catalyst surface will be more than the bulk fluid temperature. Therefore, the observed rate will be higher or lower than corresponding bulk fluid conditions, depending on both heat transfer and mass transfer effects. Rate at the surface will be higher due to heat transfer limitations and lower due to mass transfer limitations. Depending on the relative magnitude, the overall rate will be increased or decreased.
10. If a species appears in the numerator of the rate law, it is probably a product.
a) True
b) False
View Answer
Explanation: If a species appears in the numerator of the rate law, it is probably a reactant and if a species appears in the denominator of the rate law, it is probably adsorbed in the surface.
11. Molar flux consist of ______________
a) Molar flux = partial flux + diffusion
b) Total flux = bulk motion + diffusion
c) Molar flux = solid motion + flow rate
d) Total flux = partial flux + flow rate
View Answer
Explanation: Molar flux consists of two parts:
– Bulk motion of the fluid, BA
– Molecular diffusion flux relative to the bulk motion of the fluid produced by a concentration gradient, JA
– WA = BA + JA (total flux = bulk motion + diffusion).
12. What is the unit of heat transfer coefficient?
a) Watt m2 / k
b) Watt m2 k
c) Watt / m2 k
d) Watt / m2k2
View Answer
Explanation: Heat flux form a bulk fluid at T∞ to the surface of a spherical particle of diameter dp at TS: Newtons law of cooling:
q = h (T∞ – TS)
Where, q (Heat flux) = Watt / m2
h (Heat transfer coefficient) = Watt / m2K.
13. What is the unit of mass transfer coefficient?
a) ms
b) m/s
c) ms2
d) ms-2
View Answer
Explanation: Molar flux form a bulk fluid at concentration CAb to the surface of a spherical particle of diameter dp at concentration CAs:
WA = kC (CAb-CAs)
Where, WA (Molar flux) = moles/m2s
kC (Mass transfer coefficient) = m/s.
14. The ratio of momentum diffusivity (kinematic viscosity) and mass diffusivity is referred as:
a) Nusselt number
b) Schimdt number
c) Sherwood number
d) Reynolds number
View Answer
Explanation: Schmidt number (Sc) is a dimensionless number defined as the ratio of momentum diffusivity (kinematic viscosity) and mass diffusivity, and is used to characterize fluid flows in which there are simultaneous momentum and mass diffusion convection processes.
15. The ratio of inertial forces to viscous forces is called as ________
a) Nusselt number
b) Schimdt number
c) Sherwood number
d) Reynolds number
View Answer
Explanation: The Reynolds number is the ratio of inertial forces to viscous forces and is a convenient parameter for predicting if a flow condition will be laminar or turbulent. It can be interpreted that when the viscous forces are dominant (slow flow, low Re) they are sufficient enough to keep all the fluid particles in line, and then the flow is laminar. Even very low Re indicates viscous creeping motion, where inertia effects are negligible. When the inertial forces dominate over the viscous forces (when the fluid is flowing faster and Re is larger) then the flow is turbulent.
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Bioprocess Engineering Books
- Apply for Biotechnology Internship
- Check Biotechnology Books
- Practice Biotechnology MCQs