This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Yields in Cell Culture”.
1. Which of the following type of yield is referred to as “Maximum possible yields”?
a) Instantaneous yields
b) Theoretical yields
c) Observed yields
d) Non- instantaneous yields
View Answer
Explanation: Theoretical yields are sometimes referred to as maximum possible yields because they represent the yield in the absence of competing reactions.
2. The equation for aerobic production of acetic acid from ethanol is ____________
C2H5OH(ethanol) + O2(acetic acid) → CH3CO2H + H2O
Acetobacter aceti bacteria are added to vigorously-aerated medium containing 10 g l-1 ethanol. After some time, the ethanol concentration is 2 g l-1 and 7.5 g l-1 acetic acid is produced. Calculate the Observed yield.
a) 0.94 g g-1
b) 0.95 g g-1
c) 0.92 g g-1
d) 0.96 g g-1
View Answer
Explanation: Using a basis of I litre, the observed yield over the entire culture period is obtained from equation:
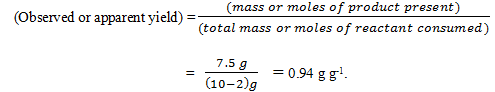
3. Refer to Q2 and, calculate the theoretical yield.
a) 1.20 g g-1
b) 1.10 g g-1
c) 1.30 g g-1
d) 1.40 g g-1
View Answer
Explanation: Theoretical yield is based on the mass of ethanol actually used for synthesis of acetic acid. From the stoichiometric equation:
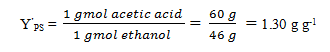
4. How many grams of carbon is/are in one mole?
a) 1 gram
b) 2 grams
c) 12 grams
d) 100 grams
View Answer
Explanation: A sample of 12 grams of carbon is equal to one mole. The amount of moles in a substance can be determined using that substance’s molar mass. The molar mass is the amount of grams in one mole of a substance. The molar mass is the average atomic mass for a substance.
5. What is the molarity of 245.0 g of H2SO4 dissolved in 1.000 L of solution?
a) 2.408 M
b) 2.508 M
c) 2.598 M
d) 2.498 M
View Answer
Explanation: MV = grams / molar mass
(x) (1.000 L) = 245.0 g / 98.0768 g mol-1
x = 2.49804235 M
to four sig figs, 2.498 M.
6. What is the molarity of 5.30 g of Na2CO3 dissolved in 400.0 mL solution?
a) 0.150 M
b) 0.125 M
c) 0.155 M
d) 0.120 M
View Answer
Explanation: MV = grams / molar mass
(x) (0.4000 L) = 5.30 g / 105.988 g mol-1
0.12501415 M
x = 0.125 M (to three sig figs).
7. What is the unit of specific growth rate?
a) g-1
b) g/h
c) gl/h
d) h-1
View Answer
Explanation: Specific growth rate = μ = \(\frac{1}{X} \frac{dX}{dt}\)
where, X = cell mass concentration (g/L)
t = time (h).
8. What do you mean by the term “Ks”?
a) Saturation constant
b) Half saturation constant
c) Variable shape constant
d) Solution constant
View Answer
Explanation: Ks is the half-saturation constant or shape factor of the Monod equation. Ks equals the substrate concentration (mg/L) at which μ equals 1/2 of μmax.
9. The yield coefficient is not used in growth kinetic relationship of which of the following growth kinetics?
a) Zero order kinetics
b) First order kinetics
c) Second order kinetics
d) Monod’s kinetics
View Answer
Explanation: The yield coefficient, commonly referred to as the substrate-to-biomass yield, is used to convert between cell growth rate dX/dt and substrate utilization rate dS/dt. The yield coefficient and the specific growth rate used to develop three types of microbial growth kinetic relationships; Monod, first order, and zero order kinetics.
10. What is the yield coefficient if the initial substrate concentration is 10 g/l and biomass is 0.1 g/l. The substrate is then consumed and produces 5.3 g/l of biomass?
a) 0.50 g biomass / g substrate
b) 0.52 g biomass / g substrate
c) 0.54 g biomass / g substrate
d) 0.56 g biomass / g substrate
View Answer
Explanation: Yield = (5.3 – 0.1 g biomass) / (10 g substrate) = 0.52 g biomass/g substrate.
11. What is the yield coefficient if the initial substrate concentration is 20 g/l and biomass is 0.2 g/l. The substrate is then consumed and produces 6.0 g/l of biomass?
a) 0.09 g biomass / g substrate
b) 0.19 g biomass / g substrate
c) 0.20 g biomass / g substrate
d) 0.29 g biomass / g substrate
View Answer
Explanation: Yield = (6.0 – 0.2 g biomass) / (20 g substrate) = 0.29 g biomass / g substrate.
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Biotechnology Books
- Check Bioprocess Engineering Books
- Apply for Biotechnology Internship
- Practice Biotechnology MCQs
