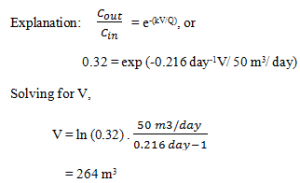This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Solving Unsteady – State Mass Balances”.
1. A compound dissolves in water at a rate proportional to the product of the amount of undissolved solid and the difference between the concentration in a saturated solution and the actual solution; i.e., Csat – C(t). A saturated solution of this compound contains 40 g per 100 g of water. In a test run starting with 20 kg of undissolved compound in 100 kg of water, it was found that 5 kg dissolved in 3 hr. if the test continues, how many kg of compound will remain undissolved after 7 hr? Assume that the system is isothermal.
a) 11.56 kg
b) 10.72 kg
c) 11.76 kg
d) 10.52 kg
View Answer
Explanation: Definitions of variables used:
C = kg dissolved compound/ kg water Cs = saturated
m = mass of undissolved solid mo = initial mass of solid
W = kg of water
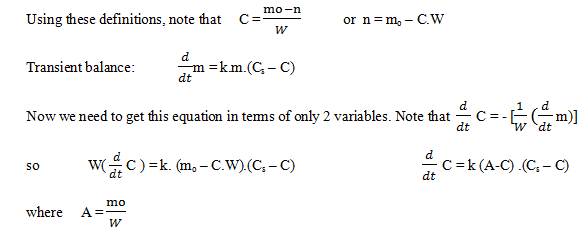
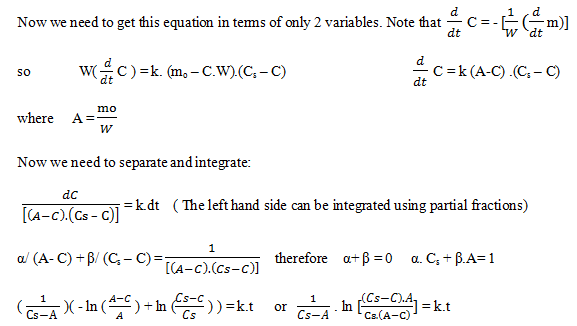
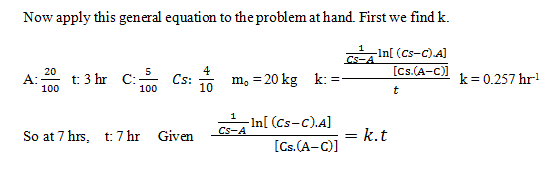
C7= 0.093 and m: mo – C7.100 kg
m = 10.72 kg.
2. What is the first – order decay?
a) The rate of loss of the pollutant is constant
b) The rate of loss of the reactant is constant
c) The rate of loss of the pollutant is directly proportional to its concentration
d) The rate of loss of the reactant is directly proportional to its concentration
View Answer
Explanation: First – order decay is the rate of loss of the pollutant is directly proportional to its concentration:
|dc/dt|reaction = -kC
For such a pollutant, mreaction = – V kC.
3. The water level in a municipal reservoir has been decreasing steadily during a dry spell, and there is a concern that the drought could continue for another 60 days. The local water company estimates that the consumption rate in the city is approximately 107 L/day. The state conservation service estimates that rainfall and stream drainage into the reservoir coupled with evaporation from the reservoir should yield a net water input rate of 106 exp (-t/100) L/day, where t is the time in days from the beginning of the drought, at which time the reservoir contained an estimated 109 liters of water.
Integrate the balance to calculate the reservoir volume at the end of the 60 days of continued drought.
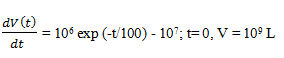
a) 4.50 × 109 L
b) 4.50 × 108 L
c) 4.45 × 108 L
d) 4.45 × 109 L
View Answer
Explanation: We now separate variables and integrate the differential balance equation from t=0 to t= 60 days.
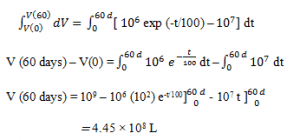
4. Which one process is not a transient process?
a) Fed- batch
b) Batch
c) Continuous
d) Semi- batch
View Answer
Explanation: Batch, semi-batch and continuous are transient as when they are start up, shut down or become transient at other times due to planned or unexpected changes in operating conditions.
5. Which of the following is the steady state condition based on the water tank concept?
a) Qin ≠ Qout
b) Qin = Qout
c) Qin > Qout
d) Qin < Qout
View Answer
Explanation: Water flows in = Water flows out;
If Qin = Qout: Steady state (No accumulation)
No increase in level, mass, volume etc.
In time, there is no change in the system.
6. Determine the volume required for a PFR to obtain the degree of pollutant reduction, Assume that the flow rate and first-order decay rate constant are unchanged (Q = 50 m3/day, k = 0.216 day-1), Cout / Cin = 32/100 = 0.32.
a) 204 m3
b) 234 m3
c) 254 m3
d) 264 m3
View Answer
7. In a batch process, the reaction takes place in the presence of an acid medium. The acid is drained from the reaction vessel at the rate of 15ml/s as a result of the density difference of the acid from the reacting component. To avoid wastage of acid, it is recycled to an acid tank which has 1000 L capacity. The acid drained from the reaction vessel, picks up 50 g/L solids from the reactor. Acid is fed once again to the process from acid tank. When the process is started, the acid is almost pure in the tank as a result of filtration. As the reaction proceeds, acid in the tank gets more and more contaminated with the solids. The concentration of the solids should not exceed 100 g/L from the process point of view. The batch time is 16h. Estimate whether the concentration of the solids will exceed 100g/L during the batch reaction.
a) 37.04 h
b) 30.05 h
c) 36.04 h
d) 32.05 h
View Answer
Explanation: Input of the solids to the tank = 15 (c+50) t∆g/1000
Output of solids from tank = 15 Δt c / 1000g
Accumulation in the tank = 1000 (c+Δc) – 1000c
= 1000Δc g
Input – output = Accumulation
1/1000 [15 (c+50) Δt – 15cΔt] = 1000Δt
750 Δt = 1000000 Δc
Converting into differential form ,
dt = 1333.33dc, when t=0 and c=0.
Integration therefore yields,
t = 1333.33c
C = 100g/L is the limit
t = 100 × 1333.33
= 133.33 s
= 37.04 h.
8. Which of the following is not an example of batch process?
a) Cooking
b) Specialty chemicals
c) Refining
d) Brewing
View Answer
Explanation: Refining is a continuous process as feed and products flow continuously through process. System is open, and usually modeled as steady flow. Examples: Petroleum refining (except coking, blending).
9. Due to algal growth in a water supply reservoir in a neighborhood, the water has acquired an unpleasant taste. The compound is non-degradable, and the water utility has decided that the best way to deal with the situation is simply to flush the contaminant out of the system. The reservoir contains 11,000 m3 of water, and the proposal is to pump 300 m3/h of clean water through the system and discharge the effluent to the sewer system until 95% of the offending compound has been removed. If the reservoir is intensely mixed, how much flushing water will be required?
a) 30, 000 m3
b) 33, 000 m3
c) 35, 000 m3
d) 39, 000 m3
View Answer
Explanation: The compound is non-reactive and therefore behaves as a tracer. The contaminant concentration in the flushing water is zero. When 95% of the contaminant has been flushed out, the concentration will be 5% of the initial concentration, so:
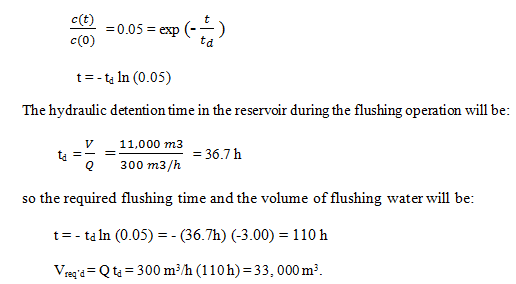
10.” A balloon is filled with air at a steady rate.” Which process can be correct for this condition?
a) Semi – batch
b) Batch
c) Fed – batch
d) Continuous
View Answer
Explanation: Semibatch Process is neither batch nor continuous process. A process is operating at a steady state if the values of all the variables in the process do not change with time, except for small fluctuations about a constant mean values. A transient or unsteady-state exists when the process variables change with time. Furthermore, batch and semibatch processes are transient operations, and continuous process can be steady-state or transient.
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Bioprocess Engineering Books
- Practice Biotechnology MCQs
- Apply for Biotechnology Internship
- Check Biotechnology Books