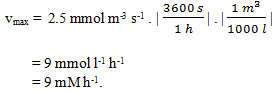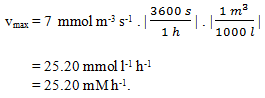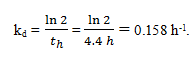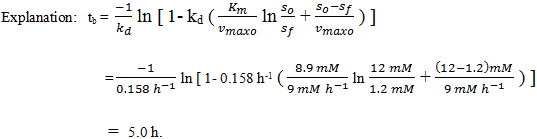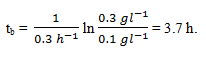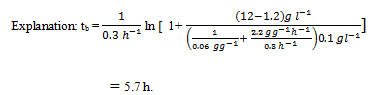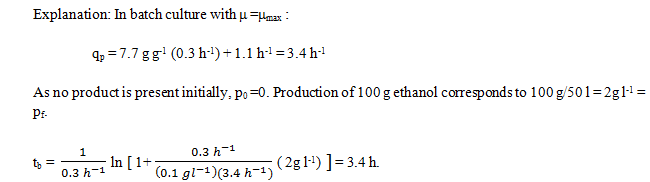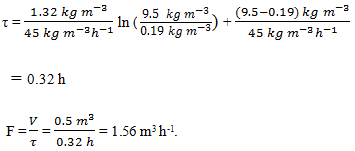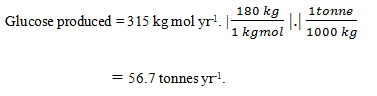This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Ideal Reactor Operation”.
1. Aerobic reactions are not batch operations.
a) True
b) False
View Answer
Explanation: Aerobic reactions are not batch operations in the strictest sense; the low solubility of oxygen in aqueous media means that it must be supplied continuously while carbon dioxide and other off-gases are removed.
2. In a perfectly mixed reactor _________
a) The output composition is different from input composition
b) The output composition is identical from input composition
c) Both output and input composition are constant
d) Both output and input composition are not constant
View Answer
Explanation: In a perfectly mixed reactor, the output composition is identical to composition of the material inside the reactor, which is a function of residence time and rate of reaction.
3. Convert vmax = 2.5 mmol m-3 s-1 into mM h-1.
a) 2500
b) 900
c) 25
d) 9
View Answer
4. Convert vmax = 7 mmol m-3 s-1 into mM h-1.
a) 25
b) 20
c) 25.20
d) 20.25
View Answer
5. In batch reaction time with enzyme deactivation, calculate the first-order deactivation rate constant.
(Given – so = 12 mM; vmax = 9 mM h-1; Km = 8.9 mM; sf = 1.2 mM; th = 4.4 h).
a) 0.150 h-1
b) 0.158 h-1
c) 0.155 h-1
d) 0.154 h-1
View Answer
6. By taking the parameters of Q5, and the batch reaction time tb.
a) 5.0 h
b) 10.0 h
c) 15.0 h
d) 20.0 h
View Answer
7. Zymomonas mobilis is used to convert glucose to ethanol in a batch fermenter under anaerobic conditions. The yield of biomass from substrate is 0.06 g g-1; YPX is 7.7 g g-1. The maintenance coefficient is 2.2 g g-1 h-1; the specific rate of product formation due to maintenance is 1.1 h-1. The maximum specific growth rate of Z. mobilis is approximately 0.3 h-1.5 g bacteria are inoculated into 50 litres of medium containing 12 g l-1 glucose. Determine batch culture times required to produce 10 g biomass.
a) 3.1 h
b) 3.3 h
c) 3.5 h
d) 3.7 h
View Answer
Explanation: Yxs = 0.06 gg-l; Ypx= 7. 7 gg-l; μmax = 0. 3 h-l; ms=2.2 gg-l h-l; mp= 1.1 h-l; x0=5g/501= 0.1 gl-l;s0= 12 gl-l.
If 10 g biomass are produced by reaction, the final amount of biomass present is (10 + 5) g = 15 g. Therefore xf = 15 g / 50 l = 0.3 g l-1.
8. By taking the pararmeters of Q7 and Determine batch culture times required to achieve 90% substrate conversion.
a) 5.1 h
b) 5.3 h
c) 5.5 h
d) 5.7 h
View Answer
9. By taking the parameters question 8 and question 7, determine batch culture times required to produce 100 g ethanol.
a) 3.2 h
b) 3.4 h
c) 3.6 h
d) 3.8 h
View Answer
10. The Zymomonas mobilis cells are used for chemostat culture in a 60 m3 fermenter. The feed contains 12 g l-1 glucose; Ks for the organism is 0.2 g l-1. What flow rate is required for a steady-state substrate concentration of 1.5 g l-1?
a) 15.6 m3 h-1
b) 15.8 m3 h-1
c) 15.4 m3 h-1
d) 15.2 m3 h-1
View Answer
Explanation: Yxs = 0.06 g g-1; Ypx = 7.7 g g-1;μmax = 0.3 h-1; Ks = 0.2 g-1; ms = 2.2 g g-1 h-l; si = 12 g 1-1; V=60 m3. qp = 3.4 h-1, YPS = Ypx Yxs = 0.46 g g-1.
s = 1.5 g l-1
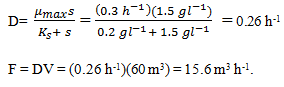
11. By taking Q10 and at the flow rate of Q10, what is the cell density?
a) 0.42 g l-1
b) 0.44 g l-1
c) 0.46 g l-1
d) 0.48 g l-1
View Answer
Explanation: When synthesis of product is coupled with energy metabolism as for ethanol, x is evaluated. Therefore:

12. By taking Q10 and Q11 into account and at the flow rate of Q10, what concentration of ethanol is produced?
a) 5.1 g l-1
b) 5.3 g l-1
c) 5.5 g l-1
d) 5.7 g l-1
View Answer
Explanation: Assuming ethanol is not present in the feed, PI = 0. Steady-state product concentration is given by:
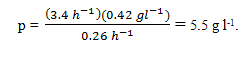
13. Which of the following type is of the perfusion culture?
a) Batch
b) Conc. Batch
c) Continuous
d) Conc. Fed-Batch
View Answer
Explanation: Concentrated Fed-Batch- In a process that can be considered a form of perfusion, the culture is perfused to generate ultrahigh cell concentration, greater than 108 cells/mL; and the product is also retained in the vessel.
14. Immobilised lactase is used to hydrolyse lactose in dairy waste to glucose and galactose. Enzyme is immobilised in resin particles and packed into a 0.5 m3 column. The total effectiveness factor for the system is close to unity; Km for the immobilised enzyme is 1.32 kg m-3; Vmax is 45 kg m-3 h-1. The lactose concentration in the feed stream is 9.5 kg m-3; a substrate conversion of 98% is required. The column is operated with plug flow for a total of 310 d per year. At what flow rate should the reactor be operated?
a) 1.56 m3 h-1
b) 1.58 m3 h-1
c) 1.50 m3 h-1
d) 1.54 m3 h-1
View Answer
15. By taking the parameters of Q14 into consideration, estimate how many tonnes of glucose are produced per year?
a) 56.3 tonnes yr-1
b) 56.6 tonnes yr-1
c) 56.7 tonnes yr-1
d) 56.5 tonnes yr-1
View Answer
Explanation: The rate of lactose conversion is equal to the difference between inlet and outlet mass flow rates of lactose:
F(si – sf) = 1.56 m3 h-1 (9.5 – 0.19) kg m-3 = 14.5 kg h-1
Converting this to an annual rate based on 310 d per year and a molecular weight for lactose of 342:
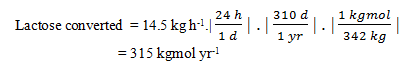
The enzyme reaction is:
lactose + H20 → glucose + galactose.
Therefore, from reaction stoichiometry, 315 kg mol glucose are produced per year. The molecular weight of glucose is 180; therefore:
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Biotechnology Books
- Practice Biotechnology MCQs
- Apply for Biotechnology Internship
- Check Bioprocess Engineering Books