This set of Chemical Reaction Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Packed Bed Catalytic Reactor – Adsorption Mechanisms”.
1. State true or false.
Physisorption occurs by Van-der-waal’s forces.
a) True
b) False
View Answer
Explanation: Physical adsorption is due to intermolecular Van-der-waal’s forces. Heat of adsorption is small and the adsorption is complete reversible.
2. The variation of Chemisorption with Pressure is ____
a) Chemisorption increases with increase in pressure
b) Chemisorption decreases with increase in pressure
c) Chemisorption increases with decrease in pressure
d) Change of pressure has no effect on Chemisorption
View Answer
Explanation: Chemisorption increases with increase in temperature, but change in pressure has no effect. The chemical adsorption is irreversible.
3. Which of the following is not an assumption of Langmuir model?
a) Energy of adsorption of gas molecules to the adsorbent site is different at different adsorbing site
b) Maximum amount of adsorption corresponds to a monolayer
c) Desorption rate depends on the amount of material adsorbed onto the solid surface
d) Gas phase molecules are adsorbed on discrete points on the solid surface
View Answer
Explanation: The energy of adsorption is same for all the sites on the adsorbent surface. The adsorption of a gas molecule at one site is independent of the adsorption on an adjacent site.
4. According to Langmuir adsorption model, the rate of adsorption of a gas molecule onto the solid surface is ____
a) Proportional to the square of the partial pressure of the gas
b) Proportional to the partial pressure of the gas
c) Inversely proportional to the square of the partial pressure of the gas
d) Inversely proportional to the partial pressure of the gas
View Answer
Explanation: The rate of adsorption of the gas molecules onto the solid surface = kads pA(1 – \(\frac{V}{V_m}\))
Where, kads is the rate constant for gas adsorption on to the solid surface
pA partial pressure of gas component ‘A’
V is the volume on the solid surface occupied by ‘A’
Vm is the volume of the entire monolayer.
5. If n is a constant for a particular gas adsorption, the dependence of \(\frac{V}{V_m}\) to the partial pressure of adsorbed gas by Freundlich adsorption is ____
a) \(\frac{V}{V_m}\) ∝ pA\(^\frac{n}{2} \)
b) \(\frac{V}{V_m}\) ∝ pA\(^\frac{1}{n} \)
c) \(\frac{V}{V_m}\) ∝ pAn
d) \(\frac{V}{V_m}\) ∝ pA
View Answer
Explanation: The volume fraction of the solid occupied by the gas is proportional to pA\(^\frac{1}{n} \). For adsorption to occur, n > 1.
6. A gas phase adsorption reaction is reaction controlling. The reaction follows Eley – Rideal mechanism, represented by A + B → R. If the product also adsorbs, which of the following rate expressions is true?
a) -rA = \(\frac{kP_A P_B}{1+ k_A P_A+ k_R P_R} \)
b) -rA = \(\frac{kP_A P_B}{(1+ k_A P_A+ k_R P_R)^2} \)
c) -rA = \(\frac{kP_A P_B}{1+ k_A P_A+ k_R P_R} \)
d) -rA = \(\frac{kP_A P_B P_R}{(1+ k_A P_A+ k_R P_R)^2} \)
View Answer
Explanation: Of the two reactant gases, A is adsorbed to the solid surface and B is in gas phase. The product R is also adsorbed. Then the rate, -rA = \(\frac{kP_A P_B}{1+ k_A P_A+ k_R P_R}. \)
7. The plot representing the variation of rate with partial pressure for a second order irreversible reaction following Eley – Rideal mechanism is ____
a) 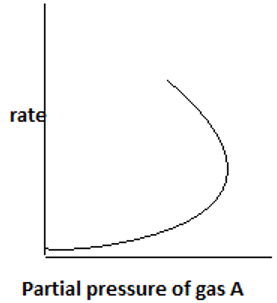
b) 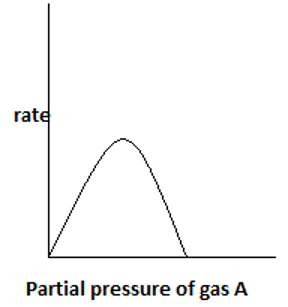
c) 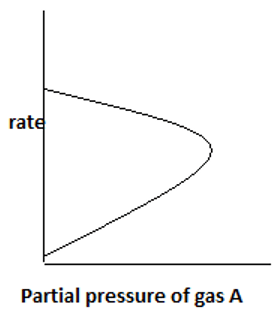
d) 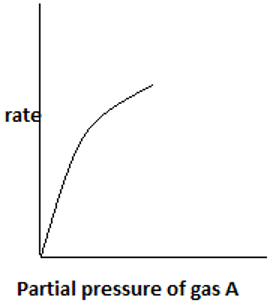
View Answer
Explanation: With an increase in partial pressure of A, more reactant gas gets adsorbed on the catalyst surface. Only one reactant component gets adsorbed and the other remains in gas phase.
8. The Langmuir Hinshelwood mechanism for equimolar reactant feed is ____
a) \(\frac{aP}{(1+bP)^2}\) = -r0
b) \(\frac{P}{(1+bP)^2}\) = -r0
c) \(\frac{aP}{(bP)^2}\) = -r0
d) \(\frac{aP}{(1+bP)}\) = -r0
View Answer
Explanation: A + B → R
If \(\frac{A}{B}\)=1, yA = yB = 0.5 for equimolar feed
-r0 = \(\frac{kP_A P(0.5)(0.5)}{(1+(0.5k_A P)+(0.5k_B P))^2}\) = \(\frac{aP}{(1+bP)^2}. \)
Where, a and b are constants, P is the total pressure.
9. The sequence of steps in a heterogeneous catalytic reaction is ____
a) Diffusion → Adsorption → Reaction → Desorption → Diffusion → Mass transfer → Mass transfer
b) Mass transfer → Diffusion → Adsorption → Reaction → Desorption → Diffusion → Mass transfer
c) Mass transfer → Diffusion → Adsorption → Desorption → Diffusion → Mass transfer → Reaction
d) Mass transfer → Adsorption → Diffusion Reaction → Desorption → Diffusion → Mass transfer
View Answer
Explanation: The catalytic reaction occurs by the mass transfer to the catalytic surface, followed by the diffusion of the reactant gas through the product layer. Reaction occurs at the gas – catalyst interface. Diffusion of the product gas occurs through the product layer. Mass transfer of the product gas occurs back through the gas film.
10. For the reaction step to be the controlling mechanism in a catalytic reversible reaction, which of the following is valid?
a) Rate of adsorption > Rate of desorption
b) Rate of adsorption < Rate of desorption
c) Rate of adsorption = Rate of desorption
d) Rate of adsorption ≠ Rate of desorption
View Answer
Explanation: If rate of adsorption of gas equals its rate of desorption, the mechanism is in equilibrium. Reaction becomes the controlling mechanism.
Sanfoundry Global Education & Learning Series – Chemical Reaction Engineering.
To practice all areas of Chemical Reaction Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Biotechnology Internship
- Apply for Chemical Engineering Internship
- Check Biotechnology Books
- Practice Biotechnology MCQs
- Practice Chemical Engineering MCQs
