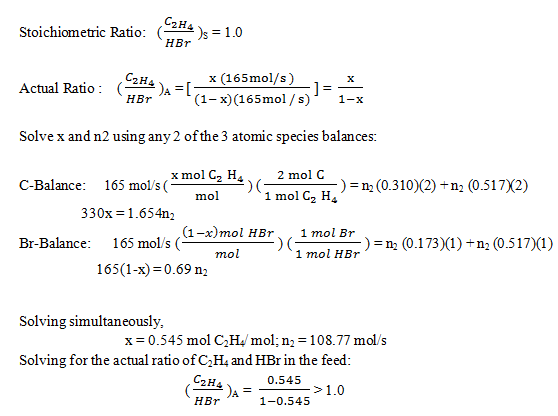This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Material Balance Numericals”.
1. If air consists of 77% by weight of nitrogen and 23% by weight of oxygen calculate:
(i) the mean molecular weight of air,
(ii) the mole fraction of oxygen,
(iii) the concentration of oxygen in mole/m3 and kg/m3 if the total pressure is 1.5 atmospheres and the temperature is 25 °C.
a) 29.8, 0.21, 0.010 mole/m3
b) 28.8, 0.21, 0.013 mole/m3
c) 27.6, 0.30, 0.010 mole/m3
d) 29.8, 0.30, 0.013 mole/m3
View Answer
Explanation: (i) Taking the basis of 100 kg of air: it contains 77/28 moles of N2 and 23/32 moles of O2,
Total number of moles = 2.75 + 0.72 = 3.47 moles.
So mean molecular weight of air = 100 / 3.47 = 28.8
Mean molecular weight of air = 28.8
(ii) The mole fraction of oxygen = 0.72 / (2.75 + 0.72) = 0.72 / 3.47 = 0.21
Mole fraction of oxygen = 0.21
(iii) In the gas equation, where n is the number of moles present: the value of R is 0.08206 m3 atm/mole K and at a temperature of 25 7deg;C = 25 + 273 = 298 K, and where V= 1 m3
pV = nRT
and so, 1.5 x 1 = n x 0.08206 x 298 n = 0.061 mole/m3
weight of air = n x mean molecular weight = 0.061 x 28.8 = 1.76 kg / m3
and of this 23% is oxygen, so weight of oxygen = 0.23 x 1.76 = 0.4 kg in 1 m3
Concentration of oxygen = 0.4kg/m3 or 0.4 / 32 = 0.013 mole / m3
When gas is dissolved in a liquid, the mole fraction of the gas in the liquid can be determined by first calculating the number of moles of gas using the gas laws, treating the volume as the volume of the liquid, and then calculating the number of moles of liquid directly.
2. In the carbonation of a soft drink, the total quantity of carbon dioxide required is the equivalent of 3 volumes of gas to one volume of water at 0⁰C and atmospheric pressure. Calculate (i) the mass fraction and (ii) the mole fraction of the CO2 in the drink, ignoring all components other than CO2 and water.
Basis 1 m3 of water = 1000 kg
Volume of carbon dioxide added = 3 m3
From the gas equation, pV = nRT
1 x 3 = n x 0.08206 x 273 n = 0.134 mole.
Molecular weight of carbon dioxide = 44
And so weight of carbon dioxide added = 0.134 x 44 = 5.9 kg
a) 6.9 × 10-3, 3.41 × 10-3
b) 8.9 × 10-3, 2.41 × 10-3
c) 5.9 × 10-3, 2.41 × 10-3
d) 9.9 × 10-3, 3.41 × 10-3
View Answer
Explanation: (i) Mass fraction of carbon dioxide in drink = 5.9 / (1000 + 5.9) = 5.9 × 10-3
(ii) Mole fraction of carbon dioxide in drink = 0.134 / (1000/18 + 0.134) = 2.41 × 10-3.
3. Each year 80,000 people move into a city, 70,000 people move out, 18,000 are born, and 11,000 die. Write a balance on the population of the city.
a) 17,000 P/yr
b) 15,000 P/yr
c) 20,000 P/yr
d) 22,000 P/yr
View Answer
Explanation: Let P denotes people,
Input + Generation – Output – Consumption = accumulation
80,000 P/yr + 18,000 P/yr – 70,000 P/yr – 11,000 P/yr = A(P/yr)
A = 17,000 P/yr
Each year the city’s population increases by 17,000 P/yr.
4. According to the Balances on steady-state processes, the accumulation is equal to?
a) 1
b) 0
c) 100
d) 2
View Answer
Explanation: The process is said to be operating at steady-state when all process variables do not change with time.
The accumulation term in a balance must equal to zero to ensure that the amount/mass of material in the process do not change with time.
Steady state means accumulation = 0
Input + generation – output – consumption = 0
Input + generation = output + consumption.
5. A process unit involves 3 chemical components. How many mass balances can be written?
a) 3
b) 5
c) 6
d) 4
View Answer
Explanation: We can write 4 balance equations: A total balance equation and 3 component balance equations.
Independent balances: Not all balances are independent since the total balance is the sum of all of the component balances.
Thus, the number of independent balances we can write = the number of components.
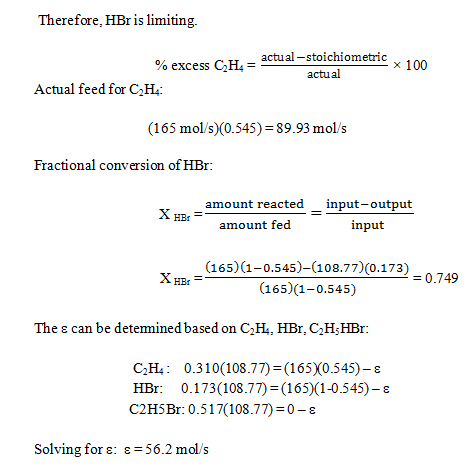
6. The reaction between ethylene and hydrogen bromide to form ethyl bromide is carried out in a continuous reactor.
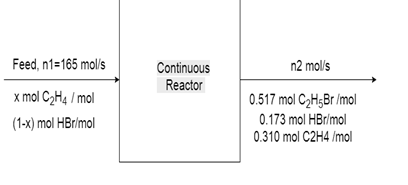
C2H4 + HBr = C2H5Br
The product stream is analyzed and found to contain 51.7 mole% C2H5Br and 17.3% HBr. The feed to the reactor contains only ethylene and hydrogen bromide.
Calculate the fractional conversion of the limiting reactant and the percentage by which the other reactant is in excess. If the molar flow rate of the feed stream is 165 mol/s, what is the extent of reaction?
a) 56.2 mol/s
b) 45.6 mol/s
c) 55.6 mol/s
d) 44.6 mol/s
View Answer
7. What is the fractional and percent excess of H2 from the following equation?
H2 + Br2 = 2HBr
H2 = 25 mol/hr
Br2 = 20 mol/hr
a) 0.45, 45%
b) 0.25, 25%
c) 0.75, 75%
d) 0.65, 65%
View Answer
Explanation: Fractional excess H2 = (25-20)/ 20 = 0.25
Percent excess = 25%.
8. When pure carbon is burned in air, some of it oxidizes into CO2 and some to CO. The molar ratio of N2 to O2 is 7.18 and the ratio of CO to CO2 is 2.0 in the product gas. What is the percent excess air used? The exit gases contain only N2, O2, CO and CO2.
a) 50 %
b) 60 %
c) 40 %
d) 20 %
View Answer
Explanation: The process involves two reactions:
R1 = C + O2 = CO2
R2 = C + 1/2 O2 = CO
As the product (output) gas composition is specified more clearly, we may use it as starting point.
Take N2 in the product as nN2 = Take N2 in the product as, then O2 = 1 mol
N2 as an inert gas: input = output = 7.18 mol
O2 input with air = 7.18 (21/79) = 1.91 mol
Total O2 consumption = 1.91-1 = 0.91 mol
If R1 uses n1 mol O2, generating n1 mol CO2, R2 uses 0.91-n1 mol O2, generating 2
(0.91-n1) mol CO.
Since CO/CO2 in the product gas = 2, 2 (0.91-n1)/n1 = 2
n1 = 0.91/2 = 0.455 mol
Therefore, total moles of C input = 3 n1 = 1.365 mol
O2 required for complete combustion of 1.365 mol C = 1.365 mol (for R1 only)
O2 excess % = (1.91 -1365)/1.365 x 100% = 39.9% = 40% .
9. Convert 800 mmHg into bars.
a) 1.064 bars
b) 1.066 bars
c) 1.054 bars
d) 1.055 bars
View Answer
Explanation: 1 mm Hg = 133.322 Pa and 1 bar = 105 Pa
Therefore,
bars = 800 mm Hg × \(\frac{133.322 \,Pa}{1 \,mm \,Hg} × \frac{1 \,bar}{10^5 \,Pa}\) = 1.066 bar.
10. What do you mean by material balance equation?
a) Original-Solids-in-place (OSIP)
b) Original-Gas-in-place (OGIP)
c) Original-Air-in-place (OAIP)
d) Original-Liquid-in-place (OLIP)
View Answer
Explanation: Material balance analysis is an interpretation method used to determine original fluids-in-place (OFIP) based on production and static pressure data. The general material balance equation relates the original oil, gas, and water in the reservoir to production volumes and current pressure conditions / fluid properties.
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Biotechnology MCQs
- Check Biotechnology Books
- Check Bioprocess Engineering Books
- Apply for Biotechnology Internship