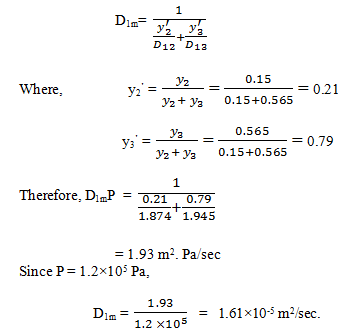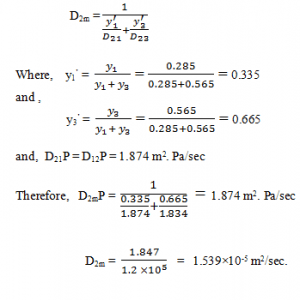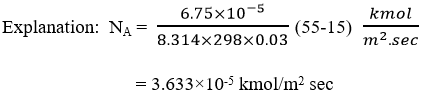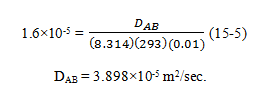This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Molecular Diffusion”.
1. In the following equation, what does y signifies?
![]()
a) Dispersion
b) Displacement
c) Diffusion
d) Distance
View Answer
Explanation: In single-phase systems, the rate of mass transfer due to molecular diffusion is given by Fick’s law of diffusion, which states that mass flux is proportional to the concentration gradient. JA is the mass flux of component A, NA is the rate of mass transfer of component A, a is the area across which mass transfer occurs, DAB is the binary diffusion coefficient or diffusivity of component A in a mixture of A and B, CA is the concentration of component A, and y is distance, \(\frac{dC_A}{dy}\) is the concentration gradient, or change in concentration of A with distance.
2. Molecular diffusion is which type of motion?
a) Thermal motion
b) Linear motion
c) Non- turbulent motion
d) Rectilinear motion
View Answer
Explanation: Molecular diffusion, often simply called diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of the particles.
3. What is Fick’s law applicable to?
a) Momentum transfer
b) Heat transfer
c) Mass transfer
d) Velocity transfer
View Answer
Explanation: In single-phase systems, the rate of mass transfer due to molecular diffusion is given by Fick’s law of diffusion, which states that mass flux is proportional to the concentration gradient.
4. Determine the diffusivity of CO2 in a gas mixture having the composition:
CO2: 28.5 %, O2: 15%, N2: 56.5%,
The gas mixture is at 273 k and 1.2 * 105 Pa. The binary diffusivity values are given as: (at 273 K)
D12 P = 1.874 m2 Pa/sec
D13 P = 1.945 m2 Pa/sec
D23 P = 1.834 m2 Pa/sec
a) 1.51×10-5 m2/sec
b) 1.50×10-5 m2/sec
c) 1.61×10-5 m2/sec
d) 1.60×10-5 m2/sec
View Answer
5. Refer to Q4, and calculate the diffusivity of O2 in a gas mixture.
a) 1.539×10-5 m2/sec
b) 1.639×10-5 m2/sec
c) 1.530×10-5 m2/sec
d) 1.630×10-5 m2/sec
View Answer
Explanation: Diffusivity of O2 in the mixture,
6. Methane diffuses at steady state through a tube containing helium. At point 1 the partial pressure of methane is pA1 = 55 kPa and at point 2, 0.03 m apart PA2 = 15 KPa. The total pressure is 101.32 kPa, and the temperature is 298 K. At this pressure and temperature, the value of diffusivity is 6.75 * 10–5 m2/sec.
Calculate the flux of CH4 at steady state for equimolar counter diffusion.
For steady state equimolar counter diffusion, molar flux is given by:
![]()
a) 3.666×10-5 kmol/m2 sec
b) 3.866×10-5 kmol/m2 sec
c) 3.833×10-5 kmol/m2 sec
d) 3.633×10-5 kmol/m2 sec
View Answer
7. Refer to Q6, and calculate the partial pressure at a point 0.02 m apart from point 1.
a) 20.63 kPa
b) 28.33 kPa
c) 28.63 kPa
d) 20.33 kPa
View Answer
8. In a gas mixture of hydrogen and oxygen, steady state equimolar counter diffusion is occurring at a total pressure of 100 kPa and temperature of 20°C. If the partial pressures of oxygen at two planes 0.01 m apart, and perpendicular to the direction of diffusion are 15 kPa and 5 kPa, respectively and the mass diffusion flux of oxygen in the mixture is 1.6 * 10–5 kmol/m2.sec, calculate the molecular diffusivity for the system.
For equimolar counter current diffusion:
![]()
a) 2.898×10-5 m2/sec
b) 3.898×10-5 m2/sec
c) 2.989×10-5 m2/sec
d) 3.989×10-5 m2/sec
View Answer
9. What is the unit of diffusion flux “J” in the Fick’s law equation?
a) mol m−2 s−1
b) mol m2 s−1
c) mol m2 s1
d) mol m−2 s1
View Answer
Explanation: J is the “diffusion flux,” of which the dimension is amount of substance per unit area per unit time, so it is expressed in such units as mol m−2 s−1. J measures the amount of substance that will flow through a unit area during a unit time interval.
10. The diffusion of gas is ____________
a) Linear process
b) Slow process
c) Spontaneous process
d) Non- spontaneous process
View Answer
Explanation: Diffusion refers to the process of particles moving from an area of high concentration to one of low concentration. The rate of this movement is a function of temperature, viscosity of the medium, and the size (mass) of the particles. Diffusion results in the gradual mixing of materials, and eventually, it forms a homogeneous mixture.
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Bioprocess Engineering Books
- Practice Biotechnology MCQs
- Check Biotechnology Books
- Apply for Biotechnology Internship