This set of Bioprocess Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Convective Mass Transfer”.
1. Convection does not occur in _______________
a) Solid
b) Liquid
c) Gas
d) Molten Solid
View Answer
Explanation: Convection cannot take place in most solids because neither bulk current flows nor significant diffusion of matter can take place. Diffusion of heat takes place in rigid solids, but that is called heat conduction. Convection, however, can take place in soft solids or mixtures where solid particles can move past each other.
2. The unit of mass transfer coefficient is ______________
a) m-1s
b) ms-1
c) m-1s2
d) m2s-1
View Answer
Explanation: (mol/s)/(m2•mol/m3) = m/s
Note, the units will vary based upon which units the driving force is expressed in. The driving force shown here as ‘ΔcA’ is expressed in units of moles per unit of volume, but in some cases the driving force is represented by other measures of concentration with different units. For example, the driving force may be partial pressures when dealing with mass transfer in a gas phase and thus use units of pressure.
3. Rate of mass transfer is inversely proportional to the rate of reaction at solid surface?
a) True
b) False
View Answer
Explanation: Estimation of the interfacial concentration CAi is more difficult; measuring compositions at phase boundaries is not easy experimentally. To overcome this problem, we must consider the processes in the system which are linked to mass transfer of A. Transport of A is linked to reaction at the surface of the solid, so that the value of CAi will depend on the rate of consumption of A at the interface. In practical terms, we can therefore calculate the rate of mass transfer of A only if we have information about the rate of reaction at the solid surface. Simultaneous reaction and mass transfer occurs in many bioprocesses.
4. In the two-phase aqueous systems, in ordinary situations the bulk phases are not in equilibrium?
a) True
b) False
View Answer
Explanation: Normally, it can be assumed that there is negligible resistance to mass transfer at the actual interface, i.e. within distances corresponding to molecular free paths on either side of the phase boundary. This is equivalent to assuming that the phases are in equilibrium at the interface; therefore, CA1i and CA2i are equilibrium concentrations. As a result it is known that there are special situations, such as when there is adsorption of material at the interface, where the assumption is invalid. However, in ordinary situations, the evidence is that equilibrium does exist at the interface between phases. If the bulk liquids were in equilibrium, no net mass transfer would take place.
5. The distribution coefficient is accurate only if?
a) Solvents are miscible
b) Solutes are miscible
c) Solvents are immiscible
d) Occurrence of chemical reaction
View Answer
Explanation: Equilibrium distribution of one solute between two phases is conveniently described in terms of the distribution law. At equilibrium, the ratio of solute concentrations in the two phases is given by the distribution coefficient or partition coefficient, m. The distribution law is accurate only if both solvents are immiscible and there is no chemical reaction.
6. In a wetted-wall tower, an air-H2S mixture is flowing by a film of water which is flowing as a thin film down a vertical plate. The H2S is being absorbed from the air to the water at a total pressure of 1.50 atm abs and 30oC. The value of kc of 9.567×10-4 m/s has been predicted for the gas-phase mass-transfer coefficient. At a given point the mole fraction of H2S in the liquid at the liquid-gas interface is 2.0×10-5 and pA of H2S in the gas is 0.05 atm. The Henry’s law equilibrium relation is pA(atm) = 609xA (mole fraction in liquid). Calculate the rate of absorption of H2S.
a) 1.480×10-3 kmol/m2.s
b) 1.486×10-3 kmol/m2.s
c) 1.485×10-3 kmol/m2.s
d) 1.487×10-3 kmol/m2.s
View Answer
Explanation: The rate of absorption of H2S per unit area of the thin film is given by:
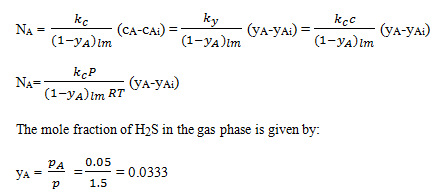
The partial pressure of H2S in the gas phase at the interface is determined from Henry’s law and the mole fraction of H2S in the liquid at the liquid-gas interface.
pAi = 609xAi = 609×2.0×10-5 = 1.218×10-2 atm
The mole fraction of H2S in the gas phase at the interface is then
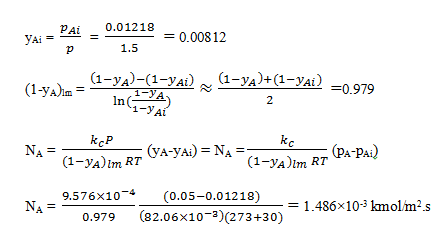
7. What do you mean by Sherwood number?
a) Dimension mass transfer number
b) Dimensionless mass transfer number
c) Dimension momentum transfer number
d) Dimensionless momentum transfer number
View Answer
Explanation: The Sherwood number (Sh) (also called the mass transfer Nusselt number) is a dimensionless number used in mass-transfer operation. It represents the ratio of the convective mass transfer to the rate of diffusive mass transport, and is named in honor of Thomas Kilgore Sherwood.
8. A stream of air at 100 kPa pressure and 300 K is flowing on the top surface of a thin flat sheet of solid naphthalene of length 0.2 m with a velocity of 20 m/sec. The other data are:
Mass diffusivity of naphthalene vapor in air = 6 * 10 –6 m2/sec
Kinematic viscosity of air = 1.5 * 10 –5 m2.sc
Concentration of naphthalene at the air-solid naphthalene interface = 1 * 10 – 5 kmol/m3
Calculate the overage mass transfer coefficient over the flat plate.
Note: For heat transfer over a flat plate, convective heat transfer coefficient for laminar flow can be calculated by the equation.
Nu = 0.664 ReL1/2 Pr1/3
you may use analogy between mass and heat transfer.
a) 0.014 m/sec
b) 0.015 m/sec
c) 0.016 m/sec
d) 0.013 m/sec
View Answer
Explanation: Given: Correlation for heat transfer
Nu = 0.664 ReL1/2 Pr1/3
The analogous relation for mass transfer is:
Sh = 0.664 ReL1/2 Sc1/3
where
Sh = Sherwood number = kL/DAB
ReL = Reynolds number = Lυρ/μ
Sc = Schmidt number = μ / (ρ DAB )
k = overall mass transfer coefficient
L = length of sheet
DAB = diffusivity of A in B
υ = velocity of air
μ = viscosity of air
ρ = density of air,
and µ/ρ = kinematic viscosity of air.
Substituting for the known quantities in equation:
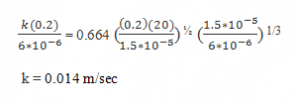
9. Refer to Q8 and, calculate the rate of loss of naphthalene from the surface per unit width.
a) 0.100 gmol/m.hr
b) 0.102 gmol/m.hr
c) 0.101 gmol/m.hr
d) 0.103 gmol/m.hr
View Answer
Explanation: Rate of loss of naphthalene = k (CAi – CA∞)
= 0.014 (1 * 10–5 – 0) = 1.4024 * 10–7 kmol/m2 sec
Rate of loss per meter width = (1.4024 * 10 –7) (0.2) = 2.8048 * 10 –8 kmol/m.sec
= 0.101 gmol/m.hr.
10. Psychrometry deals with between which type of phases?
a) Gas-liquid
b) Gas-solid
c) Gas-Vapour
d) Vapour-Solid
View Answer
Explanation: Psychrometry is concerned with the physical and thermodynamic properties of gas-vapor mixtures. Although the principles of psychrometry apply to any physical system consisting of gas-vapor mixtures, the most common system of interest is the mixture of water vapor and air, because of its application in heating, ventilating, and air-conditioning and meteorology. In human terms, our thermal comfort is in large part a consequence of not just the temperature of the surrounding air, but (because we cool ourselves via perspiration) the extent to which that air is saturated with water vapor.
11. Which type of columns are used for liquid dispersion in a continuous gas phase?
a) Packed
b) Bubble cap
c) Sieve – plate
d) Fluidized bed
View Answer
Explanation: Packed Beds. Although packed bed columns are used most often for absorption, they are also used for the distillation of vapor-liquid mixtures. The packing provides a large surface area for vapor-liquid contact, which increases the column’s effectiveness.
12. Heat transfer by molecular collision in ____________________
a) Conduction
b) Convection
c) Scattering
d) Radiation
View Answer
Explanation: Flow of heat through currents within a fluid (liquid or gas). Convection is the displacement of volumes of a substance in a liquid or gaseous phase. When a mass of a fluid is heated up, for example when it is in contact with a warmer surface, its molecules are carried away and scattered causing that the mass of that fluid becomes less dense. For this reason, the warmed mass will be displaced vertically and/or horizontally, while the colder and denser mass of fluid goes down (the low-kinetic-energy molecules displace the molecules in high-kinetic-energy states). Through this process, the molecules of the hot fluid transfer heat continuously toward the volumes of the colder fluid.
13. Convection is faster than conduction?
a) True
b) False
View Answer
Explanation: Forced air heating and air conditioning are examples of heating (or cooling) by convection. This is an effective way of bringing a hot (or cold) fluid to a different area. Convection transfers heat over a distance faster than conduction.
14. Which is the fastest mode of transfer of heat?
a) Conduction
b) Convection
c) Scattering
d) Radiation
View Answer
Explanation: Radiation is the fastest mode of transfer of heat, because radiation travels at the speed of light, which is very quick. The slowest mode of transfer of heat is conduction because it takes place from particle to particle. The fastest is radiation at the speed of light 3,00,000 km in one second.
15. Heat transfer takes place as per ______________
a) Zeroth law of thermodynamics
b) First law of thermodynamics
c) Second law of thermodynamics
d) Kirchoff’s law
View Answer
Explanation: Second Law of Thermodynamics states that It is not possible for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object.
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Bioprocess Engineering Books
- Practice Biotechnology MCQs
- Apply for Biotechnology Internship
- Check Biotechnology Books
