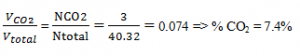This set of Bioprocess Engineering Interview Questions and Answers for Experienced people focuses on “Heat of Reaction for Processes with Biomass Production”.
1. What is the process of making biomass energy?
a) Oxidation
b) Combustion
c) Reduction
d) Vaporization
View Answer
Explanation: It is burning (combustion). The combustion process generates heat that’s transformed into energy. In the combustion process biomass is burned and converted into energy.
2. A solid-oxide fuel cell is fed with carbon monoxide and reacts with air to produce CO2. This reaction will produce 2 electrons which are used to power an electric circuit external to the fuel cell. The reaction equation is shown below:
2CO(g) + O2(g) -> 2CO2(g) ΔHr° = -565.96 kJ/mol
This reaction does not occur for other types of fuel cells which use a catalyst, such as polymerelectrolyte membrane or phosphoric-acid fuel cells. The presence of carbon monoxide on the anode side of these types of fuel cells will cause catalyst poisoning, reducing the efficiency and voltage of the fuel cell.
Determine the rate of enthalpy change for a carbon dioxide production rate of 208 mol/hr.
The extent of the reaction occurring in the fuel cell can be obtained by the following equation:
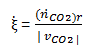
where:
a) -16.35 kW
b) -15.35 kW
c) -16.45 kW
d) -15.45 kW
View Answer
Now the rate of change in the enthalpy for the oxidation of carbon monoxide can be calculated as follows:
ΔH = ξ ̇ΔHr°
Entering the known quantities for the extent of reaction and the enthalpy of reaction into this equation yields:
ΔH ̇= (104 mol/hr) (-565.96 kJ/mol).(1hr/3600 s)
= -16.35 kJ/s
ΔH ̇= -16.35 kW.
3. Refer to Q2 and estimate that the synthesis gas obtained from a coal gasification process can be used for producing methanol, which is used as fuel in direct-methanol fuel cells. Determine the rate of production of methanol if the reaction shown below is releasing 21.6 kW of energy.
CO(g) + 2H2(g) -> CH3OH(l) ΔHr° = -128.08 kJ/mol
a) 455.6 mol/hr
b) 457.2 mol/hr
c) 450.9 mol/hr
d) 454.5 mol/hr
View Answer
Explanation: Solving for ξ ̇and substituting the corresponding quantities into this equation yields:
The rate in enthalpy change was considered to be negative since the problem is stating that the reaction is releasing energy (exothermic reaction).
Now we can enter the calculated extent of reaction into the equation previously solved for the molar production rate of methanol, to get:

4. In this problem we wish to develop the combustion equation and determine the air-fuel ratio for the complete combustion of n-Butane (C4H10) with theoretical air.
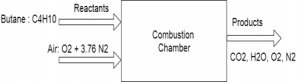
a) 15.4 kg-air/kg-fuel
b) 12.5 kg-air/kg-fuel
c) 12.4 kg-air/kg-fuel
d) 15.5 kg-air/kg-fuel
View Answer
5. Refer to Q4, and calculate 50% excess air.
a) 22.2 kg-air/kg-fuel
b) 23.2 kg-air/kg-fuel
c) 20.2 kg-air/kg-fuel
d) 20.3 kg-air/kg-fuel
View Answer
6. In this problem Propane (C3H8) is burned with 61% excess air, which enters a combustion chamber at 25°C. Assuming complete combustion and a total pressure of 1 atm (101.32 kPa), determine the air-fuel ratio [kg-air/kg-fuel].
61% Excess air (161% Theoretical air):
C3H8 + (1.61) z (O2 + 3.76 N2) => 3 (CO2) + 4(H2O) + (0.61)z (O2) + (1.61) (3.76) z (N2 )
Equating coefficients, z = 3+2 = 5 (oxygen component balance), thus:
C3H8 + 8.05(O2 + 3.76 N2) ⇒ 3(CO2) + 4(H2O) + 3.05(O2) + 30.27(N2)
a) 26.3 kg-air/kg-fuel
b) 25.5 kg-air/kg-fuel
c) 25.3 kg-air/kg-fuel
d) 26.5 kg-air/kg-fuel
View Answer
7. Refer to Q6 and calculate the percentage of carbon dioxide by volume in the products.
a) 7.0%
b) 6.0%
c) 7.4%
d) 6.4%
View Answer
8. Refer to Q6 and Q7 and calculate the dew point temperature of the products.
a) 45.8°C
b) 40.5°C
c) 30.5°C
d) 35.8°C
View Answer
Explanation: NH2O = 4, \(\frac{P_{H_{2}O}}{P} = \frac{N_{H_2O}}{N_{total}} = \frac{4}{40.32} => P_{H_2O} = (101.32 kPa)[\frac{4}{40.32}]\) = 10.05 kPa
Tdew-point = Tsat@10kPa = 45.8°C.
9. “Thermal conversion of organic matter with an oxidant (normally oxygen) to produce primarily carbon dioxide and water”, which term is used for this process?
a) Oxidation
b) Pyrolysis
c) Combustion
d) Gasification
View Answer
Explanation: Combustion is defined as thermal conversion of organic matter with an oxidant (normally oxygen) to produce primarily carbon dioxide and water. The oxidant is in stoichiometric excess, i.e., complete oxidation.
10. ”Thermal conversion (destruction) of organics in the absence of oxygen”, which term is used for this process?
a) Reduction
b) Pyrolysis
c) Combustion
d) Gasification
View Answer
Explanation: Pyrolysis is defined as thermal conversion (destruction) of organics in the absence of oxygen. In the biomass community, this commonly refers to lower temperature thermal processes producing liquids as the primary product. Possibility of chemical and food byproducts.
Sanfoundry Global Education & Learning Series – Bioprocess Engineering.
To practice all areas of Bioprocess Engineering for Interviews, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Bioprocess Engineering Books
- Apply for Biotechnology Internship
- Practice Biotechnology MCQs
- Check Biotechnology Books

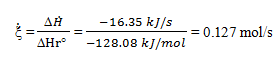
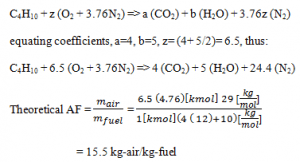
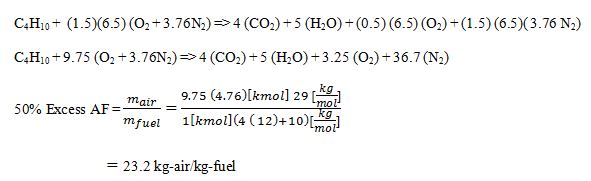
![Find the air-fuel ratio [kg-air/kg-fuel] if Propane (C3H8) is burned with 61% excess air](https://www.sanfoundry.com/wp-content/uploads/2018/03/bioprocess-engineering-questions-answers-heat-reaction-processes-biomass-production-q6-300x104.png)
![The air-fuel ratio [kg-air/kg-fuel] 25.3 kg-air/kg-fuel for oxygen component balance](https://www.sanfoundry.com/wp-content/uploads/2018/04/bioprocess-engineering-questions-answers-heat-reaction-processes-biomass-production-q6b.png)