This set of Molecular Endocrinology Multiple Choice Questions & Answers (MCQs) focuses on “Nuclear Receptor Signaling Mechanisms”.
1. What is the proper range of domains in nuclear receptors?
a) A to F
b) A to D
c) F to M
d) M to Z
View Answer
Explanation: Nuclear receptors are proteins with molecular masses ranging from 50,000 to 100,000 d. Nuclear receptors have a similar structure, with a highly variable amino-terminal domain and a central DNA-binding domain that is conserved. They share a similar domain sequence, referred to as proper domains A through F.
2. In which domain range is the nuclear localization signal (NLS) located?
a) A and B
b) C and D
c) A and D
d) D and F
View Answer
Explanation: The nuclear receptors are synthesized on ribosomes that exist outside the nucleus, like all cellular proteins. The nuclear localization signal (NLS), which is located near the boundary of the C and D domains, is needed to import nuclear receptors into the nucleus.
3. How do the glucocorticoid receptor exist in the absence of the hormone?
a) Tethered in the endoplasmic Reticulum with chaperones
b) Tethered in the nucleus with chaperones
c) Tethered in the cytoplasm with chaperones
d) Tethered in the cytoplasm with chaperones
View Answer
Explanation: In the absence of a hormone, the glucocorticoid receptor is bound to a network of chaperone molecules in the cytoplasm, namely heat shock proteins. The binding of hormones to the glucocorticoid receptor triggers a conformation transition that results in chaperone dissociation.
4. Much of the nuclear receptors exist in the nucleus as a result of their NLSs.
a) True
b) False
View Answer
Explanation: The nuclear receptor superfamily of transcription factors regulates gene expression by interacting with target genes through DNA sequence-specific interactions. The majority of nuclear receptors exist in the nucleus as a result of their NLSs, with or without their ligands. The glucocorticoid receptor is a significant exception.
5. Nuclear receptors have the capacity to recognize and bind to the gene subset that their cognate ligand can regulate.
a) True
b) False
View Answer
Explanation: Nuclear receptors are highly specific in their binding actions. One such specificity is the binding of nuclear receptors to their cognate ligand-specific gene subsets. They regulate transcription by interacting with the unique DNA sequences of these cognate ligand-specific genes called elements of hormone reaction (HREs).
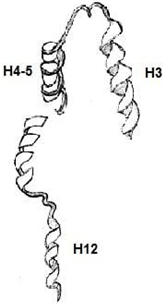
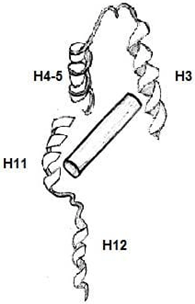
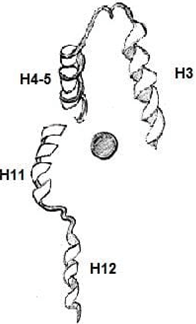
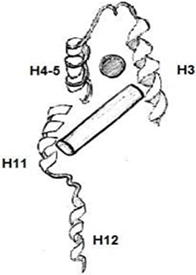
6. Which among the following is an Apo-Receptor (no ligand bound)?
a)
b)
c)
d)
View Answer
Explanation: Apo-receptors are receptors that targets apoproteins or apolipoproteins. A lipophilic ligand’s high-affinity binding is a shared feature of many nuclear receptors. When a ligand is bound there is a conformational change happening in the receptor.
7. Which domain mediate the High-affinity binding of a lipophilic ligand?
a) D and E
b) A and B
c) B and C
d) C and D
View Answer
Explanation: High-affinity binding of a lipophilic ligand is a shared characteristic of many nuclear receptors. This defining function of the receptor is mediated by the C-terminal ligand-binding domain (LBD), domains D and E.
8. What is the similar overall structure shared by the ligand binding domains (LBD)?
a) 15-beta helical segments
b) 15-alpha helical segments
c) 12-beta helical segments
d) 12-alpha helical segments
View Answer
Explanation: The LBD structure for a variety of receptors has been discovered recently. All have a common general structure, consisting of 12 alpha-helical segments in a tertiary structure that is strongly folded.
9. In which of the three hydrophobic segments does the ligand binds in a nuclear receptor?
a) H3, H4 and H5
b) H3, H4 and H6
c) H2, H4 and H5
d) H3, H7 and H5
View Answer
Explanation: In helices 3, 4, and 5 (H3, H4, and H5, respectively), the ligand binds inside a hydrophobic pocket consisting of amino acids. The internal folding of most C-terminal helix (H12), which forms a cap on the ligand-binding pocket, is the main structural change caused by ligand binding.
10. Which among the following is the sequence into which the steroid hormone receptors bind?
a) AGCTCA
b) AGAACA
c) AGGTCG
d) AATTCC
View Answer
Explanation: AGAACA is the sequence into which the steroid hormone receptors specifically bind. However, estrogen receptor doesn’t bind to this sequence as an exception. All such sequences are in the 5’ to 3’ direction and they are composed of specific purines or pyrimidines.
11. What is the factor that determine the sequence specificity of hormone binding?
a) Lipid residues in the P box
b) Protein residues in the P box
c) Amino acid residues in the P box
d) Peptide residues in the P box
View Answer
Explanation: A set of amino acid residues in the P box of the DBD is the main determinant of hormone binding specificity. These hexamer DNA sequences are referred to as half-sites. In the case of steroid/thyroid hormone receptors, the three P-box amino acids in the DNA recognition-helix help to differentiate the central base pairs of the hexameric half-sites of receptor response elements in DNA.
12. The major dimerization domain in steroid receptors is present in which domain?
a) A domain
b) B domain
c) C domain
d) D domain
View Answer
Explanation: In steroid receptors, the major dimerization domain is inside the C domain, while LBD contributes to it. Dimerization and DNA binding of steroid hormone receptors is facilitated by ligand binding.
13. What heterodimerization interaction of the receptor takes place even without DNA?
a) DBD mediated
b) LBD mediated
c) PPAR mediated
d) AGAR mediated
View Answer
Explanation: Two different interactions mediate heterodimerization with RXR, one involving LBDs and the other involving DBDs. The best interaction that happens even in the absence of DNA is mediated by the receptor LBD.
14. Where do VDR/RXR heterodimers bind preferentially?
a) Direct Repeats
b) Tandem Repeats
c) Short sequences
d) Analogue signals
View Answer
Explanation: The RXR/VDR DNA complex is a key member of the RXR heterodimer class that binds to DRs with 2, 3, 4, or 5 nucleotide spacers. VDR/RXR heterodimers, separated by three bases, preferentially bind to Direct Repeats (DRs). DR4 binds to TR/RXR, and DR5 binds to RAR/RXR with the highest affinity.
15. What region of the receptor take part in recognition of hexamer spacing in steroid hormone nuclear receptors?
a) C domain
b) A domain
c) B domain
d) D domain
View Answer
Explanation: Recognition of hexamer spacing is carried out by C domain. This domain forms heterodimers with the RXR. The nucleus, cytosol, and plasma membrane of target cells all contain steroid hormone receptors.
Sanfoundry Global Education & Learning Series – Molecular Endocrinology.
To practice all areas of Molecular Endocrinology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
