This set of Molecular Endocrinology Multiple Choice Questions & Answers (MCQs) focuses on “Insulin”.
1. Which among the following cells are unresponsive to insulin hormone?
a) RB cells
b) Muscle cells
c) Liver cells
d) Adipose tissues
View Answer
Explanation: The muscles, liver, adipose tissue and heart are the main target tissues of insulin. RB cells, epithelial cells of the GI tract, and renal tubular epithelial cells usually do not respond to insulin. Human erythrocytes are highly specialized cells whose function is to transport oxygen. The sole metabolic energy source of these cells is the fermentation of glucose by glycolysis.
2. What happens to insulin hormone when its disulfide bond is broken with some reducing agents?
a) Activated
b) Inactivated
c) Released
d) Stored
View Answer
Explanation: Insulin is inactivated by the breakage of disulfide bonds with alkali or reduction agents. Insulin protein digestion with proteolytic enzymes also inactivates the hormone. For the receptor binding activity of insulin, all three disulfide bonds are important, while the various disulfide bonds make various contributions to the overall insulin structure.
3. Where is the intrachain di-sulphide bridge located in the ‘A’ chain of the insulin structure given below?
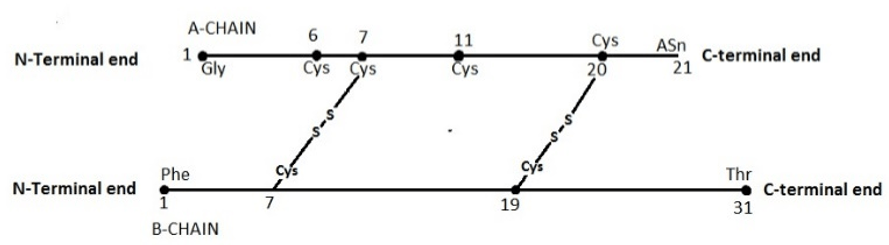
a) Between Cys7 and Cys11
b) Between Cys11 and Cys20
c) Between Cys6 and Cys11
d) Between Gly1 and Cys11
View Answer
Explanation: ‘A’ chain bears an S-S “intra-chain” link between Cys6 and Cys11. Two S-S linkages hold both A chain and B chain together. Although the amino acid sequence of insulin varies between species, the positions of the three disulfide bonds, both ends of the A chain and the C-terminal residues of the B chain, are strongly conserved in some segments of the molecule.
4. Insulin is an important anabolic hormone.
a) True
b) False
View Answer
Explanation: Insulin plays an important function in metabolism, inducing absorption of carbohydrates, accumulation of glycogenesis and glycogen, synthesis of fatty acids or storage of triglycerols, and uptake of amino acids or synthesis of proteins. Therefore, insulin is a major anabolic hormone that works on various tissues.
5. Pro-insulin when compared to other forms of insulin is the most active form.
a) True
b) False
View Answer
Explanation: Pro-insulin is biologically inactive, although it can cross-react with anti-sera prepared against insulin. This form of insulin is made up of 86 amino acids. Proinsulin is an insulin prohormone precursor produced in the beta cells of the specialized regions of the pancreas of the islets of Langerhans.
6. The difference between human insulin and porcine insulin is located in which amino acid?
a) Number 30 amino acid of A chain
b) Number 30 amino acid of B chain
c) Number 20 amino acid of A chain
d) Number 20 amino acid of B chain
View Answer
Explanation: Porcine insulin is identical to the insulin in humans. Only the terminal amino acid number 30 in the B-chain varies. It is alanine in place of threonine in the porcine insulin. Patients who have transitioned to human insulin have reported substantial reductions in the levels of anti-insulin antibodies, making insulin allergies easier to control.
7. Which type of porcine insulin is used in the treatment of diabetes mellitus?
a) De alaninated
b) De glycinated
c) De valinated
d) Threonine present
View Answer
Explanation: Porcine Insulin, or pig insulin, is the most studied hormone and is a two-chain polypeptide hormone formed in vivo in pancreatic beta cells. The elimination of alanine (de-alaninated) maintains the biological function of porcine insulin. Due to the lower antigenicity, de-alaninated insulin has been used in treating diabetes mellitus.
8. Which among the following is the correct order of formation of insulin?
a) Pre proinsulin > Pro Insulin
b) Pre proinsulin > Insulin
c) Pro insulin > Pre proinsulin > insulin
d) Pre proinsulin > Pro insulin > Insulin
View Answer
Explanation: In the beginning pre-proinsulin is produced during insulin biosynthesis, and is transformed to pro-insulin. After that the latter is converted to insulin by the action of certain set of enzymes. Only in beta cells in the pancreas is the insulin synthesized in large amounts.
9. Which enzyme acts on pro insulin to convert it to insulin in the diagram given below?
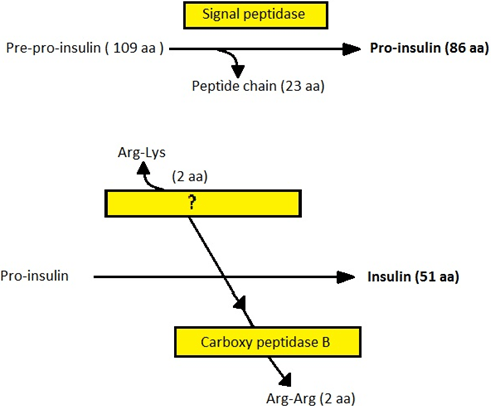
a) Trypsin like protease
b) Signal peptidase
c) Glycine like protease
d) Alanine like protease
View Answer
Explanation: Proinsulin is acted on by a trypsin-like protease in the Golgi cisternae that hydrolyses the peptide chain at two positions. As a result, an inert connective ‘C’ peptide is activated and the A-and-B chain is left with two active peptide chains.
10. Which enzyme is otherwise known as insulinase?
a) Proteases
b) Glutathione-insulin transhydrogenase
c) Trypsin like proteases
d) Peptidases
View Answer
Explanation: Insulinase is another name of the enzyme glutathione-insulin transhydrogenase. This enzyme is present in the liver and kidneys at the highest concentrations. High levels of zinc and slightly lower levels of manganese produce human insulinase.
11. Which are the two enzymes that take part in insulin catabolism?
a) Peptidase and Glutathione-insulin transhydrogenase
b) Lipases and Glutathione-insulin transhydrogenase
c) Proteases and Glutathione-insulin transhydrogenase
d) Isomerases and Glutathione-insulin transhydrogenase
View Answer
Explanation: Insulin is a major regulator of the metabolism of glucose, lipids, and proteins. It is a hormone which is catabolized very easily. Two enzyme mechanisms are involved in insulin degradation. They are proteases and the enzyme Glutathione-insulin transhydrogenase.
12. Which among the following cells have a higher concentration of the protease enzymes involved in insulin degradation?
a) Muscles
b) Bones and embryo
c) Brain and RBC
d) Liver and kidney
View Answer
Explanation: In several tissues, insulin-specific proteases have been identified, where the largest concentration is seen in the liver and kidneys. At physiological pH, the protease is SH-dependent and active. A protease is an enzyme that catalyzes (increases the rate of) proteolysis, the breakdown of proteins into smaller polypeptides or single amino acids, called peptidase or proteinase.
13. Human insulin gene receptor is found on which chromosome?
a) Chromosome 19
b) Chromosome 18
c) Chromosome 21
d) Chromosome 15
View Answer
Explanation: The gene for the human insulin receptor is present on chromosome 19. The insulin receptors are increasingly being synthesized and degraded. The INS gene provides guidance for the development of the hormone insulin that is essential to regulate blood glucose levels.
14. What happens to the insulin receptors when the concentration of insulin increases in the blood?
a) Decreases
b) Increases
c) Inactivates
d) Activates
View Answer
Explanation: The number of insulin receptors on the target cell membrane is reduced by a high level of blood insulin. This is achieved by bringing the insulin-receptor complex into the cell and thus reducing the sensitivity of the target tissues to insulin.
15. What is the name of the hypothesis which describes the reciprocal relationship between cAMP and cGMP in the action of insulin?
a) Wobbler hypothesis
b) JAK-KINASE hypothesis
c) Yin Yang hypothesis
d) Reciprocal hypothesis
View Answer
Explanation: When insulin binds to a particular receptor, multiple events of action take place along with the activity of cAMP and cGMP. The reciprocal relationship in the function of cyclic AMP and cyclic GMP is known as Yin Yang hypothesis.
Sanfoundry Global Education & Learning Series – Molecular Endocrinology.
To practice all areas of Molecular Endocrinology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
