This set of Molecular Endocrinology Multiple Choice Questions & Answers (MCQs) focuses on “Receptor Tyrosine Kinases – Set 2”.
1. Why the insulin hormone receptor is not active even if it is dimerized?
a) Two halves not oriented properly
b) Absence of receptor domain
c) Absence of DNA binding domain
d) Absence of ligand binding domain
View Answer
Explanation: In the insulin receptor, the molecular information appears to be explored. However, the two halves of the insulin receptor are not optimally oriented to allow activation of the receptor in the absence of a ligand.
2. Which among the following is the insulin receptor kinase?
a) Tyr1172
b) Tyr1162
c) Tyr1189
d) Tyr9983
View Answer
Explanation: The insulin receptor kinase is Tyr1162. Tyr1162 is situated in such a position in the inactive form of the insulin receptor kinase so that it prevents protein substrates from binding to the active site.
3. How do insulin activate its receptor for metabolic activities?
a) Binding with DNA binding domain
b) Dephosphorylation of the tyrosine residues
c) Phosphorylation of the tyrosine residues
d) Binding with ligand binding domain
View Answer
Explanation: Insulin binding causes autophosphorylation in the activation loop of three tyrosine residues (Tyr1158, Tyr1162, and Tyr1163). A major conformational transition happens as these three tyrosine residues become phosphorylated.
4. The capacity to bind insulin is maintained by alpha-beta monomers, but is not activated in response to insulin binding.
a) True
b) False
View Answer
Explanation: In many case, multiple studies have shown that for insulin to activate its receptor, receptor dimerization is required. Alpha-beta monomers, maintain insulin binding potential but are not activated in response to insulin binding.
5. As the ligand binds to the extracellular domain, it activates the activity of the intracellular domain of tyrosine kinase.
a) True
b) False
View Answer
Explanation: As the ligand binds to the extracellular domain, the intracellular domain activity of tyrosine kinase is stimulated. Although the detailed mechanisms of transmembrane signaling are not entirely known, significant progress has been made in finding receptor activation molecular mechanisms.
6. Which among the following is the conserved motif to which the insulin receptor substrate (IRS) binds?
a) Asn-Pro-Xaa-PLys
b) Asn-Pro-Xaa-pTyr
c) Asn-Pro-Xaa-PSer
d) Asn-Pro-Xaa-pVal
View Answer
Explanation: A strongly maintained phosphotyrosine-binding (PTB) domain that binds to a conserved motif (Asn-Pro-Xaa-pTyr) characterizes the insulin receptor substrate (IRS) proteins. Phosphorylation of the Tyr residue in the Asn-Pro-Xaa-pTyr motif involves the attachment of the PTB domain to the insulin receptor.
7. Which among the following is the highly conserved domains seen in certain tyrosine kinases that bind phosphotyrosine residues?
a) SRC homology 2 (SH2)
b) SRC homology 3 (SH3)
c) SRC homology 4 (SH4)
d) SRC homology 5 (SH5)
View Answer
Explanation: The substrates for certain tyrosine kinases include the domain of SRC homology 2 (SH2). These are strongly conserved domain binding residues of phosphotyrosine. A structurally conserved protein domain found within the Src oncoprotein and in many other intracellular signal-transducing proteins is the SH2 (Src Homology2) domain.
8. Which among the following is an example of the co-localized plasma protein of insulin receptor?
a) pp120/hepatocyte antigen 7 (HA7)
b) pp120/hepatocyte antigen 6 (HA6)
c) pp120/hepatocyte antigen 5 (HA5)
d) pp120/hepatocyte antigen 4 (HA4)
View Answer
Explanation: Co-localization has the capacity to facilitate phosphorylation. The insulin receptor was identified to phosphorylate pp120/hepatocyte antigen4 (HA4). The insulin receptor is a plasma membrane transmembrane protein where extracellular insulin is detected and signals are transmitted into the cellular signalling network.
9. Which receptor is targeted to the plasma membrane by an amino terminal myristoylation site?
a) FRS2
b) FRS3
c) HA4
d) HA5
View Answer
Explanation: FGF receptor substrate 2 (FRS2) is a fibroblast growth factor receptor substrate. It is targeted by an amino terminal myristoylation site to the plasma membrane. The key mediator of signalling in the FGF pathway is the fibroblast growth factor (FGF) receptor substrate 2 alpha (FRS2 alpha).
10. In the diagram given below, which is the ligand that binds to the receptor for the ligand mediated activation of the given tyrosine kinase receptor?
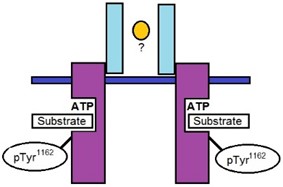
a) Glucagon
b) Insulin
c) Adrenaline
d) Androgen
View Answer
Explanation: In the activation loop, phosphorylation of tyrosine residues leads to activation of the tyrosine kinase insulin receptor. The model is based on the isolated insulin receptor tyrosine kinase three-dimensional structure, as determined by x-ray crystallography.
11. Which among the following domains, share the ability to bind pTyr residues?
a) SH2
b) SH3
c) SH4
d) SH5
View Answer
Explanation: Src homology 2 (SH2) domains are protein modules found in several proteins involved in tyrosine kinase signalling cascades (of approximately 100 amino acids). The ability to bind pTyr residues is shared by SH2 domains. However, with respect to their binding specificity, individual SH2 domains differ.
12. What determines the binding affinity of the SH2 domains?
a) Three amino acid residues downstream
b) DNA binding domain
c) Conserved sequences
d) Mattifying domains
View Answer
Explanation: The binding affinity of a domain of SH2 is determined by the three residues of amino acids downstream from the residue of pTyr. SH2 phosphatidylinositol 3 kinase (PI3K) domains, for example, exhibit a preference for pTyr-(Met/Xaa)-Xaa-Met.
13. Which among the following is the correct amino acid residue that determines the specificity of GRB2?
a) pTyr-Xaa-Asn-Xaa
b) pTyr-(Met/Xaa)-Xaa-Met
c) pTyr-(Met/Xaa)-Xaa-Asn
d) pTyr-Xaa-Asn-Met
View Answer
Explanation: An adaptor protein involved in signal transduction/cell communication is growth factor receptor-bound protein2, also known as Grb2. The growth factor receptor-binding protein 2 (GRB2) SH2 domain favors pTyr-Xaa-Asn-Xaa binding. This amino acid specificity is different for different hormones.
14. SH3 domains bind to which amino acid rich sequence?
a) Proline
b) Valine
c) Leucine
d) Isoleucine
View Answer
Explanation: SH3 domains consist of conserved sequences that bind to proline-rich sequences (approximately 50 amino acid residues). SH3 domains are present in many proteins that function in signaling pathways, like the SH2 domains.
15. Which are the three highly conserved domains of the cellular SRC?
a) One kinase catalytic domain and two noncatalytic SH domains
b) Two kinase catalytic domain and one SH domain
c) One kinase catalytic domain, One SH domain and One DNA binding domain
d) One kinase catalytic domain and two DNA binding domains
View Answer
Explanation: As SRC’s amino acid sequence is studied, it is evident that the molecule has three closely conserved domains. They are the kinase catalytic domain and two homology domains of noncatalytic SRC that are referred to as SH2 and SH3.
Sanfoundry Global Education & Learning Series – Molecular Endocrinology.
To practice all areas of Molecular Endocrinology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
