This set of Molecular Endocrinology Multiple Choice Questions & Answers (MCQs) focuses on “Receptor Tyrosine Kinases”.
1. What is the basis for the classification of the family of receptor tyrosine kinases into subfamilies?
a) Differences in the structure of the extracellular domain
b) Differences in the number of the extracellular domain
c) Differences in the function of the extracellular domain
d) Differences in the structure of the intracellular domain
View Answer
Explanation: Among the sequences of the extracellular domains, there is significant variation. There are 16 subfamilies in the family of receptor tyrosine kinases, identified mainly on the basis of variations in extracellular domain structure.
2. What family of receptors mediate the biologic actions of a wide variety of ligands, including insulin, and other growth factors?
a) Thyroid hormone Receptors
b) Receptor Tyrosine Kinases
c) Receptor activin family
d) Protein kinase Receptors
View Answer
Explanation: The biological activities of a wide range of ligands, including insulin, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor, are regulated by receptor tyrosine kinases. The difference in the extracellular domain sequences allows the receptors to bind the ligands to this diverse collection.
3. Which among the following was the first cell surface receptor demonstrated to possess tyrosine kinase activity?
a) Vascular endothelial growth factor
b) Platelet-derived growth factor (PDGF)
c) Epidermal growth factor (EGF)
d) Insulin like growth factor
View Answer
Explanation: Epidermal growth factor is a protein that, by binding to its receptor, EGFR, promotes cell growth and differentiation. The first cell surface receptor demonstrated to possess tyrosine kinase activity was the EGF receptor. It was also the first tyrosine kinase receptor to be cloned.
4. Receptor tyrosine kinases have many structural characteristics in common.
a) True
b) False
View Answer
Explanation: There are some structural features common to Receptor tyrosine kinases. This includes an extracellular domain that includes the site of ligand binding, a single transmembrane domain, and an intracellular part that includes the catalytic domain of tyrosine kinase.
5. Of all the receptors in Receptor Tyrosine Kinase family, the tyrosine kinase domain is the most strongly conserved sequence.
a) True
b) False
View Answer
Explanation: Human genome review shows that it includes sequences for about 100 tyrosine kinases of the receptor. Of all receptors in this family, the tyrosine kinase domain is the most strongly conserved sequence.
6. What happens when a ligand binds to the receptor tyrosine kinase?
a) Inactivated
b) Dimerizes
c) Translated
d) Polymerized
View Answer
Explanation: The EGF receptor, like most receptor tyrosine kinases, occurs mostly as a monomer in the absence of a ligand. Ligand binding, however, causes receptor dimerization. Ligand binding contributes to activation of the receptor kinase function and autophosphorylation in its cytosolic domain of tyrosine residues.
7. What property of the receptor tyrosine kinases has the ability to fine-tune the specificity of receptors for ligand binding and downstream signaling?
a) Heterodimer formation
b) Homodimer formation
c) Polymer formation
d) Monomer formation
View Answer
Explanation: The EGF receptor can form heterodimers with other members of the same subfamily of receptor tyrosine kinases, in addition to the ability to form homodimers. Although a small number of receptors can be incorporated into a large number of pairings, the formation of heterodimers has the ability to fine-tune the specificity of ligand binding and downstream signaling receptors.
8. Which subunit of the pro-receptors of tyrosine kinase receptor contains the ligand binding site?
a) Delta subunit
b) Gamma subunit
c) Alpha subunit
d) Beta subunit
View Answer
Explanation: The tyrosine kinase receptors are synthesized into two subunits (α and β) as proreceptors which undergo proteolytic cleavage. The β-subunit includes the site of the ligand-binding and the transmembrane and tyrosine kinase domains are found in the β-subunit.
9. Which among the following stabilizes the α2β2 heterotetramers of insulin and IGF receptor?
a) Intersubunit disulfide bonds
b) Intrasubunit disulfide bonds
c) Covalent bonding
d) Electrostatic bonding
View Answer
Explanation: The insulin receptor closely resembles the type 1 IGF receptor, which mediates IGF1’s biological activities. Both receptors function as heterotetramers of alpha2β2, which are stabilized by Intersubunit disulfide bonds.
10. Which among the following tyrosine kinase receptor exist as a dimer even without the binding of ligand?
a) Epidermal Growth Factor Receptors (EGFR)
b) Insulin Receptor
c) Platelet Derived Growth Factor Receptor (PDGFR)
d) Vascular Endothelial Growth Factor Receptor (VEGFR)
View Answer
Explanation: It is only after ligand binding, almost all receptor tyrosine kinases are known to be dimerized. However, the insulin receptor, even in the absence of a ligand, exists as a dimer of alpha-beta monomers.
11. Which among the following is the correct structure of insulin receptor?
a)
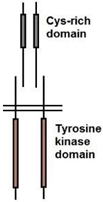
b)
![]()
c)
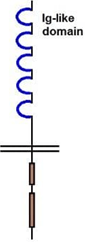
d)

View Answer
Explanation: The insulin receptor is a heterodimer dimer containing two alpha-chains and two beta-chains, represented as (alpha-beta)2. The alpha chain and about 190 residues at the N-terminal of the beta-chain are found on the extracellular side of the plasma membrane.
12. Which among the following is the receptor of Vascular Endothelial Growth Factor?
a) FLT1
b) CGF2
c) LJH3
d) CDK3
View Answer
Explanation: A potent angiogenic factor is the vascular endothelial growth factor (VEGF), which was first identified as an important growth factor for vascular endothelial cells. FLT1 is the receptor of Vascular Endothelial Growth Factor. The crystal structure of VEGF when it is bound to its receptor, FLT1, provides clear support for this form of mechanism.
13. Which among the following factors of the GH enable appropriate conformational change to activate the Growth Hormone (GH) receptor–associated tyrosine kinase, JAK2?
a) Presence of two different receptor binding sites
b) Presence of amino acid residues
c) Presence of DNA binding domain
d) Presence of catalytic domain
View Answer
Explanation: On each GH molecule, there are two distinct receptor-binding sites. This causes the ligand to bind to receptor dimers and to activate the GH receptor-associated tyrosine kinase, JAK2, triggering the necessary conformational change.
14. How are mutant ligands of the tyrosine kinase receptor created?
a) By abolishing one of the two receptor binding sites
b) By abolishing two different receptor binding sites
c) By insertional activation
d) By insertional inactivation
View Answer
Explanation: It is possible to design mutant ligands that bind to one of the two sites but lack the capacity to bind to both by abolishing one of the two receptor-binding sites. Therefore, the mutant ligands do not activate hormone activity, but they block the endogenous hormone’s action by binding to receptors.
15. Which among the following diseases can be treated with mutant growth hormones (GH)?
a) Acromegaly
b) Syphilis
c) Phenylketonuria
d) Maple Syrup Urine Disease
View Answer
Explanation: In disorders such as acromegaly, mutated GH molecules have been used for treatment purpose. Acromegaly is a hormonal disease that occurs when too much growth hormone is produced by the pituitary gland during adulthood.
Sanfoundry Global Education & Learning Series – Molecular Endocrinology.
To practice all areas of Molecular Endocrinology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
