This set of Chemical Reaction Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Catalysts”.
1. Which of the following is an example for a heterogeneous catalyst?
a) Proton
b) Rhodium and cobalt complex
c) Carbonic anhydrase
d) Platinum on alumina
View Answer
Explanation: Platinum on alumina is the heterogeneous catalyst used for production of benzene for cyclohexane. Proton is a homogenous catalyst. Carbonic anhydrase is an enzyme which acts as a homogenous catalyst. Hydroformylation is done using rhodium and cobalt complex as the homogenous catalyst.
2. What is the catalyst used for oxidation reaction?
a) CuCl2
b) Cu
c) Pd
d) Pt
View Answer
Explanation: Cu is the catalyst used for oxidation reaction. CuCl2 is used for halogenation reaction. Pd is used for alkylation reaction. Pt is used for hydrogenation reaction. Oxidation reaction is the reaction in which the reactant combines with oxygen.
3. From the diagram shown below, what does step 3 stand for?
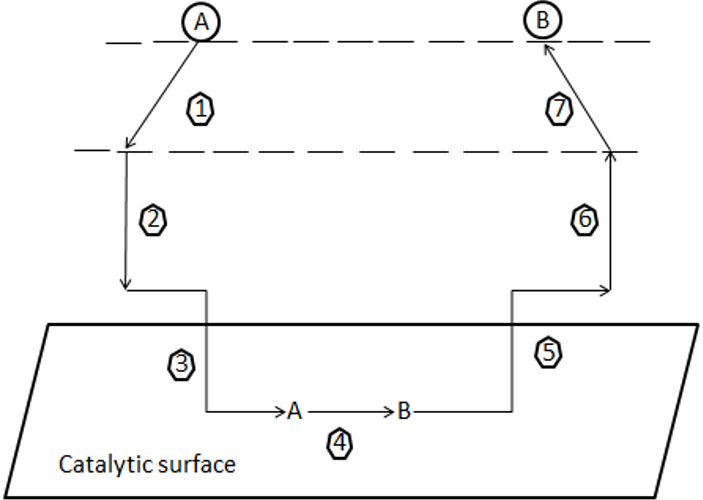
a) Adsorption of reactant A onto the catalyst surface
b) Reaction of the A on the catalyst surface
c) Desorption of A onto the catalyst surface
d) Mass transfer of the product to the catalyst surface
View Answer
Explanation: The diagram shown above represents, the steps which will take place when a reactant A undergoes a chemical reaction to form the product B with the help of heterogeneous catalysis. Adsorption is of two types, they are molecular adsorption and dissociative adsorption.
4. A catalyst will change the rate of the reaction but it will not affect the equilibrium.
a) True
b) False
View Answer
Explanation: Catalysts are used to accelerate or slow the rate of the reaction and does not have the potential to change its equilibrium. The catalyst will change the path of the reaction by reducing the activation energy.
5. The diagram shown below represents the basic unit operations in solid catalyst preparation. What does step 3, 4, and 7 stand for?
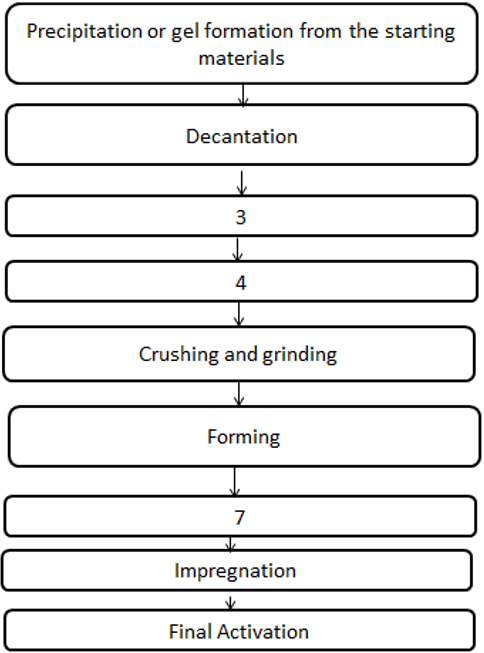
a) Calcination, drying, washing
b) Drying, washing, calcination
c) Washing, drying, calcination
d) Drying, calcination, washing
View Answer
Explanation: Generally, there are 9 steps in the preparation of a solid catalyst. Washing is done to remove the impurities that might be present after filtration. The next step, drying is done to remove the moisture content in the catalyst. Calcination is the process of heating the catalyst to high temperature.
6. In sol – gel process of catalyst preparation, what are the two possible resulting products?
a) Xerogel and pseudogel
b) Xerogel and aerogel
c) Aerogel and crystogel
d) Gel and solvent
View Answer
Explanation: Xerogel is the product obtained when the gel is dried by evaporation. Aerogel is the product obtained when the gel is dried by supercritical drying. The aerogel retains high porosity and has higher pore volume.
7. A cubical crystal was placed in an X – ray diffractometer using incoming x – rays with a wavelength of 0.145 nm. The variation of the peak intensities recorded at various 2θ values are given below
| 2θ(deg) | 50.3 | 58.3 | 76.2 | 159.3 | 136.4 |
| h k l | 101 | 200 | 231 | 401 | 312 | Relative intensity | 115 | 16 | 25 | 3 | 15 |
Given k = 0.9, β = 0.0098
What will be the crystal size?
a) 45.23 nm
b) 25.36 nm
c) 15.26 nm
d) 14.71 nm
View Answer
Explanation: The crystal size is calculated with the peak corresponding to 2θ = 50.3, which has the highest peak intensity.
We have k = 0.9, β = 0.0098, λ(wavelength) = 0.145 nm, θ = 50.3 / 2 = 25.15
Crystal size L = \(\frac {kλ}{β cosθ} = \frac {0.9*0.145}{0.0098*cos(25.15)}\) = 14.71 nm
8. For a first order chemical reaction in a porous catalyst, the Thiele modulus is 12. What will be the effectiveness factor?
a) 1
b) 1.2
c) 0.833
d) 0
View Answer
Explanation: Given, Thiele modulus = 12.
For Thiele modulus (mL) > 5, effectiveness factor is inversely proportional to the Thiele modulus.
Therefore, effectiveness factor is 1 / 12 = 0.0833.
9. Which of the following is an example of catalytic poison?
a) Potassium
b) Chloride
c) Carbon
d) Helium
View Answer
Explanation: Carbon is used as catalytic poison on silica – alumina catalyst used for cracking of petroleum. Catalyst poison is a substance that is used to deactivate catalyst wholly or partially. It will lead to chemical deactivation.
10. What is the equation of Thiele modulus?
a) L / D
b) L(k / D)0.5
c) Lk / D
d) k / D
View Answer
Explanation: Thiele modulus is ∅2 = \(\frac {surface \, reaction \, rate}{diffusion \, rate}\). Generally for a first order reaction it is ∅ = L(k / D)0.5. In the above equation L refers to the size of the particle, D refers to the diffusivity constant and k refers to the rate constant.
Sanfoundry Global Education & Learning Series – Chemical Reaction Engineering.
To practice all areas of Chemical Reaction Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Apply for Biotechnology Internship
- Practice Biotechnology MCQs
- Check Chemical Reaction Engineering Books
- Check Biotechnology Books
