This set of Corrosion Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Eight Forms – Uniform Corrosion”.
1. What is the important basis for the classification of corrosion?
a) Appearance of corroded metal
b) Nature of metal
c) Environmental conditions
d) Corrosion rate
View Answer
Explanation: The basis for the classification of corrosion is the appearance of corroded metal. Each form can be identified by visual observation as it has a unique form of appearance.
2. Which of the following is/are types of corrosion?
a) Erosion corrosion
b) Galvanic corrosion
c) Pitting corrosion
d) Erosion, galvanic and pitting corrosion
View Answer
Explanation:
Types of corrosion: i. Uniform corrosion
ii. Galvanic corrosion
iii. Crevice corrosion
iv. Pitting corrosion
v. Intergranular corrosion
vi. Selective leaching
vii. Erosion corrosion
viii. Stress corrosion
3. Which of the following type of corrosion is depicted in the given figure?
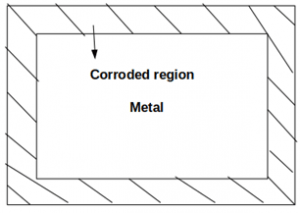
a) Pitting corrosion
b) Uniform corrosion
c) Erosion corrosion
d) Selective leaching
View Answer
Explanation: Uniform corrosion is an electrochemical corrosion in which metal undergoes uniform depth of deterioration over its entire exposed surface. Example: Rusting of iron in atmospheric air.
4. Which of the following is an example of uniform corrosion?
a) Zinc dissolution in dilute H2SO4
b) Dezincification of brass in acids
c) Rusting of iron
d) Zinc dissolution in dilute H2SO4 and rusting of iron
View Answer
Explanation: Uniform corrosion is characterized by an electrochemical reaction that proceeds uniformly over the entire exposed surface. Zinc dissolution in dilute H2SO4 and rusting of iron are examples of uniform corrosion. Dezincification of brass in acids is an example of selective leaching.
5. Tarnishing of silver is an example of which type of corrosion?
a) Crevice corrosion
b) Pitting corrosion
c) Uniform corrosion
d) Erosion corrosion
View Answer
Explanation: Outermost layers of silver undergoes a chemical reaction with hydrogen sulfide results in tarnish. This is known as the tarnishing of silver. Metals like brass, copper, aluminum, silver, and magnesium undergoes tarnishing under various corrosive environments.
6. What is/are the preventions of uniform corrosion?
a) Proper material coatings
b) Inhibitors
c) Cathodic protection
d) Proper material coatings, inhibitors, and cathodic protection
View Answer
Explanation: Uniform corrosion can be prevented by proper material coatings, inhibitors, and cathodic protection. Proper material coatings minimize the exposure area of metal. An inhibitor is a chemical substance that decreases the corrosion rate of metal by modifying the surface of anode or cathode.
7. Which of the following is/are the causes of catastrophic failures of corrosion?
a) Incomplete weld penetration
b) Extremely porous casting
c) Improper heat treatment
d) Incomplete weld penetration, extremely porous casting and improper heat treatment
View Answer
Explanation: Corrosion is an electrochemical reaction that results in the degradation of a material. Corrosion failures are usually catastrophic in nature. And the causes are incomplete weld penetration, extremely porous casting, and improper heat treatment.
8. What is the standard electrode potential of Fe+2/Fe?
a) 0.0 V
b) +0.36
c) -0.44
d) -0.76
View Answer
Explanation: Standard reduction potential of hydrogen ion, copper, iron, and zinc Is 0.0V, +0.36V, -0.44V and -0.76V respectively. These are measured against the standard hydrogen electrode (SHE) which arbitrarily defined as zero.
9. Pure metals are more corrosion resistant than commercial materials.
a) True
b) False
View Answer
Explanation: In most of the cases of corrosion, pure metals are more corrosion resistant than commercial materials. This is due to the absence of galvanic effect and selective leaching in pure metals.
10. Which of the following are the types of metallic coatings that prevent corrosion?
a) Metal cladding
b) Electroplating
c) Metal coat extrusion
d) Metal cladding, electroplating and metal coat extrusion
View Answer
Explanation: Corrosion is an electrochemical reaction that results in the degradation of a material. It can be prevented by various metal coating methods such as metal cladding, electroplating and metal coat extrusion.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Mechanical Engineering Internship
- Check Corrosion Engineering Books
- Check Mechanical Engineering Books
- Check Metallurgical Engineering Books
- Apply for Metallurgical Engineering Internship
