This set of Corrosion Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Modern Theory Principles – Passivity”.
1. Which of the following is/are correct regarding passivation?
a) It is defined as the loss of reactivity at certain conditions
b) Usually results in the formation of the oxide layer
c) The corrosion rates are relatively low
d) It is defined as loss of reactivity at certain conditions usually due to the formation of the oxide layer and corrosion rates are relatively low
View Answer
Explanation: Passivation is defined as the loss of reactivity of metals/alloys at certain conditions usually due to the formation of the oxide layer and corrosion rates are relatively low.
2. The passive state of a metal is often relatively unstable and subject to damage.
a) True
b) False
View Answer
Explanation: Passivation is the loss of chemical reactivity of a metal due to the formation of the oxide layer. It is relatively unstable and subject to damage. Chemical properties, wear resistance and thickness are the factors for the stability of the passive layer.
3. Which of the following is/are the metal/alloys that demonstrate active-passive transitions?
a) Iron and stainless steels
b) Nickel and its alloys
c) Iron, stainless steels, nickels, titanium, and its alloys
d) Titanium and its alloys
View Answer
Explanation: Metals/alloys that demonstrate active-passive transitions is/are:
4. What is depicted in the given polarization curve?
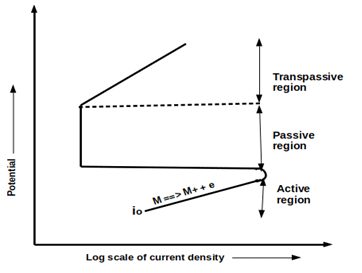
a) Cathodic dissolution of typical active-passive transition metal
b) Anodic dissolution of typical active-passive transition metal
c) Anodic dissolution of a metal
d) Cathodic dissolution of a metal
View Answer
Explanation: Given the polarization curve, depicts the typical anodic dissolution of active-passive transition metal. It includes an active region, passive region, and trans passive region.
5. Which of the following is/are correct regarding the active region of metals?
a) Corrosion rates increase exponentially
b) Corrosion rates increase linearly
c) It follows typical Tafel behavior
d) It follows typical Tafel behavior and corrosion rates increases exponentially
View Answer
Explanation: Active region is the first stage of active-passive transition in which the corrosion rates increase exponentially and it follows typical Tafel behavior.
6. The Trans passive region is the region in which the corrosion rate increases with an increase in potential.
a) True
b) False
View Answer
Explanation: Trans passive region is the last region of active-passive transitions of metal in which the corrosion rate increases with an increase in potential. It is formed due to the instability of the passive layer at that high potentials.
7. Which of the following is/are the important characteristics of active-passive transitions of a metal?
a) Passive potential (Epp)
b) Critical anodic current density (ic)
c) Passive potential (Epp) and Critical anodic current density (ic)
d) Neither passive potential nor critical anodic current density
View Answer
Explanation: Passive potential is defined as the potential of a system at which the passivation of metal is starts whereas critical anodic current density is defined as the current density at which passivation of metals starts.
8. Which of the following statements is/are correct regarding the given schematic?
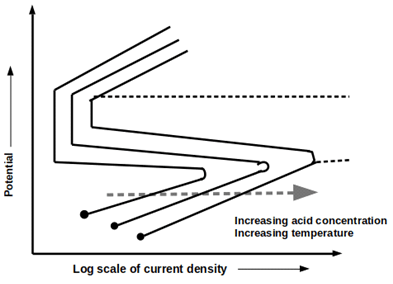
a) Increase in acid concentration and temperature increase the passive potential
b) Increase in acid concentration and temperature increase critical current density
c) The relative reduction in the size of the passive region
d) Increase in acid concentration and temperature increase critical current density, passive potential and we can observe a relative reduction in the size of the passive region
View Answer
Explanation: Statements that are correct regarding the given schematic are:
9. Which of the following metal doesn’t possess a trans passive region?
a) Iron
b) Titanium
c) Nickel
d) Aluminum
View Answer
Explanation: Typical active-passive transitions metals possess active region, passive region, and trans passive region. Whereas titanium doesn’t possess trans passive region is due to the formation of a more protective titanium oxide film structure.
10. Which of the following metals/alloys passivates spontaneously in acid solutions containing oxidizers or dissolved oxygen?
a) Copper
b) Titanium
c) Stainless steels and titanium
d) Nickel
View Answer
Explanation: Stainless steels and titanium passivates spontaneously in acid solutions containing oxidizers or dissolved oxygen. A decrease in passive potential and critical current density is the driving force for the spontaneous passivation.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Mechanical Engineering MCQs
- Check Metallurgical Engineering Books
- Check Mechanical Engineering Books
- Practice Metallurgical Engineering MCQs
- Apply for Metallurgical Engineering Internship
