This set of Corrosion Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Mineral Acids – Sulfuric Acid”.
1. Which of the following is/are the examples of mineral acids?
a) Sulfuric acid and nitric acid
b) Hydrochloric acid and hydrofluoric acid
c) Sulfuric, nitric, hydrochloric, hydrofluoric and phosphoric acid
d) Sulfuric, nitric, hydrochloric and hydrofluoric acid
View Answer
Explanation: Mineral acid or inorganic acid is the acid derives from one or more inorganic compounds on dissolution with water. This includes sulfuric acid, nitric acid, hydrochloric acid, hydrofluoric acid, and phosphoric acid.
2. Which of the following acid is produced relatively higher than other acids?
a) Nitric acid
b) Sulfuric acid
c) Phosphoric acid
d) Hydrofluoric acid
View Answer
Explanation: Sulfuric acid is produced relatively higher than other acids. The chemical formula for sulfuric acid is H2SO4. It is produced by absorbing water into oleum.
3. What are the principal uses of sulfuric acid?
a) Production of hydrochloric acid
b) Manufacturing of dyes, fertilizers, and drugs
c) Pickling of steels and other metals
d) Production of hydrochloric acid, manufacturing of dyes, fertilizers and drugs and pickling of steels
View Answer
Explanation: Principle uses of sulfuric acid are:
- Production of hydrochloric acid and other chemicals
- Manufacturing of dyes, fertilizers, drugs, explosives, and rayon
- Pickling of steels and other metals
- Laboratory usage.
4. Which of the following method is used to produce sulfuric acid?
a) Contact process
b) Ostwald process
c) Solvay process
d) Haber process
View Answer
Explanation: Sulfuric acid is produced by the contact process or leads chamber process. It is produced by absorbing SO3 with water. This process accounts for 70% of world production.
5. Which of the following statements is/are true regarding the contact process?
a) Conversion of sulfur dioxide to sulfur trioxide takes place
b) Vanadium oxide is used as a catalyst
c) It accounts for 70% of the world’s production
d) Conversion of sulfur dioxide to sulfur trioxide takes place in the presence of vanadium catalyst and it accounts for 70% world’s production
View Answer
Explanation: Contact process:
6. Which of the following concentration of sulfuric acid in which the corrosion rate of steel is minimum?
a) Less than 30%
b) Greater than 70%
c) 30% – 70%
d) above 110%
View Answer
Explanation: Ordinary steels are widely used for various concentrations of sulfuric acid greater than 70% purity. Dilute acids attack steel very rapidly and result in faster degradation.
7. Which of the following are the functions of the given graph on the x and y-axis respectively?
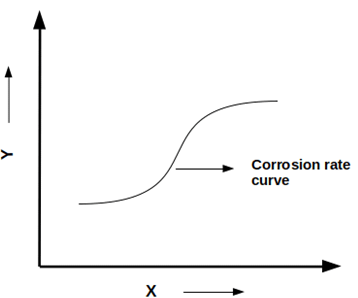
a) Temperature and concentration of acid
b) Corrosion rate and temperature
c) The concentration of acid and temperature
d) The concentration of acid and corrosion rate
View Answer
Explanation: The given graph is an isocorrosion chart. It is a streamlined method devised to present the corrosion data. The concentration of acid and temperature are the functions of the isocorrosion chart on the x and y-axis respectively.
8. Which of the following is/are the factors that affect the corrosion resistance of steel in concentrated sulfuric acid?
a) High velocity
b) Temperature of acid
c) Aeration
d) High velocity, temperature, and aeration
View Answer
Explanation: High velocity, the temperature of acid and aeration are the factors that affect the corrosion resistance of steel in concentrated sulfuric acid (>70%).
9. Which of the following metal is not corrosion resistant to sulfuric acid?
a) Grey cast iron
b) Duriron
c) Durimet 20
d) Lead
View Answer
Explanation: Grey cast iron is not corrosion resistant to sulfuric acid as it penetrates the metal along with the graphite flakes. Though the corrosion rates are small but the metal may split open in service.
10. Lead is used extensively for sulfuric acid in the lower concentration ranges (<70%).
a) False
b) True
View Answer
Explanation: Lead is used extensively for sulfuric acid in the lower concentration ranges (<70%). It is not recommended above 70% purity is due to the solubility of lead sulfate surface film in the acid.
11. Which of the following is/are the applications of Duriron because of its corrosion resistance and inherent hardness?
a) To manufacture pumps, valves, and fans
b) To make heat exchangers
c) Impressed–current anodes
d) To manufacture pumps, valves, fans, heat exchangers and impressed-current anodes
View Answer
Explanation: Duriron is high-silicon cast iron with high corrosion resistance and inherent hardness. It is used to manufacture pumps, valves, fans, heat exchangers, and impressed-current anodes.
12. Which of the following alloying elements are not recommended in the sulfuric acid environment?
a) Chromium
b) Nickel
c) Molybdenum
d) Nickel and molybdenum
View Answer
Explanation: Alloying elements are added in base metal to exhibit certain specific properties such as corrosion resistance, thermal properties, or mechanical properties. Here molybdenum is not recommended as an alloying element in high-silicon cast iron for sulfuric acid application.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Metallurgical Engineering Books
- Check Mechanical Engineering Books
- Apply for Mechanical Engineering Internship
- Apply for Metallurgical Engineering Internship
- Practice Metallurgical Engineering MCQs
