This set of Corrosion Engineering Questions & Answers for Exams focuses on “Modern Theory Principles – Polarization – 2”.
1. Which of the following is/are correct regarding concentration polarization?
a) Diffusion of ions in the bulk solution is the controlling factor
b) It usually operates at a high current density
c) It is a continuation of Activation polarization
d) It is a continuation of activation polarization, diffusion of ions in the bulk solution is the controlling factor and it usually operates at high current densities
View Answer
Explanation: Concentration polarization is the mechanism of polarization in which diffusion of ions in the bulk solution is the controlling factor, it is a continuation of activation polarization and it usually operates at high current densities.
2. What is depicted in the given figure?
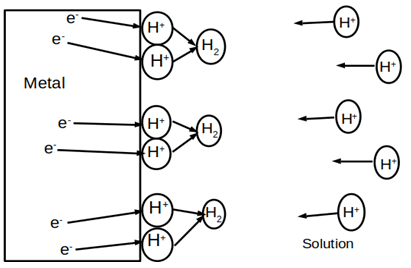
a) Activation polarization
b) Concentration polarization
c) Resistance polarization
d) Activation and concentration polarization
View Answer
Explanation: The given figure depicts the concentration polarization of a reaction. It is the mechanism of polarization in which the diffusion of ions in the bulk solution is the controlling factor. It usually encounters at high-density currents and low concentration solution.
3. What is the formula to determine limiting diffusion current density (iL) of concentration polarization?
a) iL=xnFCB/D
b) iL=DCB /nFx
c) iL=DnFCB/x
d) iL=x/DnFCB
View Answer
Explanation: iL=DnFCB/x is the formula to determine the limiting diffusion current density (iL) of concentration polarization. Where IL is the limiting diffusion current density, D is the diffusion coefficient, CB concentration of ions in bulk solution and x is the thickness of diffusion layer.
4. Agitation will increase the limiting diffusion current density of concentration polarization.
a) True
b) False
View Answer
Explanation: Agitation tends to decrease the thickness of the diffusion layer by supply enough ions to the metal interface hence there will be an increase in limiting diffusion current density of concentration polarization.
5. Which of the following is/are correct regarding the given figure?
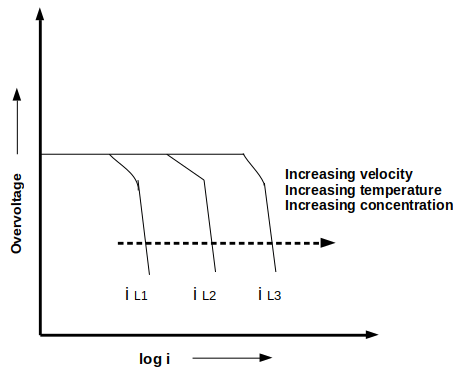
a) Velocity, temperature, and concentration are the factors that influence limiting current density
b) Limiting current density decreases with an increase in velocity, temperature, and concentration
c) Limiting current density increase with the increase in velocity, temperature, and concentration
d) Velocity, temperature, and concentration are the factors that influence limiting current density and it increases as it increases
View Answer
Explanation: The given figure shows the increase in limiting diffusion current density of concentration polarization with an increase in velocity, temperature, or concentration. It is due to the relatively high availability of reacting species at the interface and decrease in thickness of the diffusion layer.
6. Which of the following type(s) of polarization is depicted in the given figure?
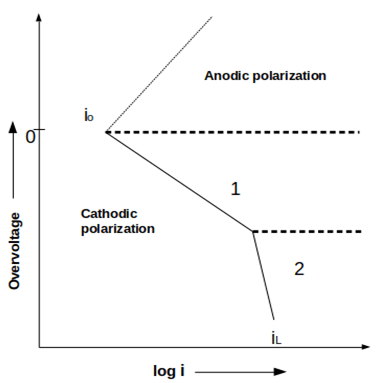
a) Activation polarization
b) Concentration polarization
c) Activation and concentration polarization
d) Resistance polarization
View Answer
Explanation: The given figure depicts the cathodic polarization of an element with activation and concentration polarizations. Concentration polarization is the continuation of activation polarization at higher current densities.
7. Which of the following is/are correct regarding resistance polarization?
a) Activation energy at the interface is the controlling factor
b) The resistance of transition between electrodes and electrolytes
c) Diffusion of ions in the bulk solution is the controlling factor
d) The resistance of reacting ions for diffusion is the controlling factor
View Answer
Explanation: Resistance polarization is the type of polarization in which there is a resistance of transition between electrodes and electrolytes. But this polarization has a negligible effect on corrosion kinetics.
8. Which two scientists present the first formal presentation on mixed potential theory?
a) Wagner and Traud
b) Wagner and Evans
c) Traud and Nernst
d) Wilsmore and Nernst
View Answer
Explanation: Mixed potential theory is the electrochemical hypothesis of modern corrosion presented by Wagner and Traud in the year 1938. It relates the potentials and currents of the corrosion system to determine corrosion potential (Ecorr) and corrosion current (icorr).
9. Which of the following is/are the hypothesis of mixed potential theory?
a) Any electrochemical reaction can be divided into two or more partial reactions
b) No net accumulation of electric charge during the reaction
c) Any electrochemical reaction can be divided into two or more partial reactions and no net accumulation of electric charge during the reaction
d) Electrochemical reactions can’t be divided into partial reactions
View Answer
Explanation: Hypothesis of mixed potential theory:
i. Any electrochemical reactions can be divided into two or more partial reactions
ii. No net accumulation of electric charge during the reaction.
10. Which of the following is depicted in the given mixed potential schematic?
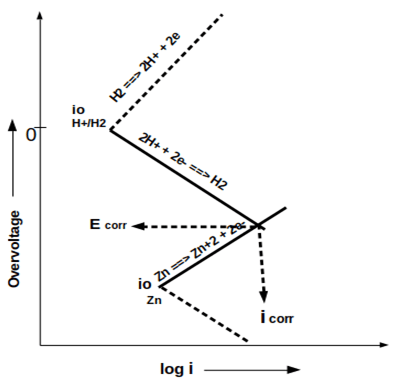
a) Dissolution of zinc
b) Oxidation of hydrogen
c) Reduction of zinc
d) Dissolution of copper
View Answer
Explanation: The given mixed potential schematic depicts the dissolution of zinc. It shows the anodic polarization of zinc, cathodic polarization of hydrogen, and resulting in Ecorr and icorr.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all exam questions on Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Metallurgical Engineering Internship
- Practice Mechanical Engineering MCQs
- Check Corrosion Engineering Books
- Apply for Mechanical Engineering Internship
- Check Metallurgical Engineering Books
