This set of Corrosion Engineering Questions and Answers for Experienced people focuses on “Principles – Metallurgical and Other Aspects”.
1. Which of the following are the types of materials, that are categorized based on its atomic arrangement?
a) Crystalline
b) Amorphous
c) Semi-crystalline
d) Crystalline, amorphous and semi-crystalline
View Answer
Explanation: The atoms in the crystalline materials are regularly arranged with the same repeating unit in a long-range order. Examples of crystalline materials are metals.
The atoms in which the atoms are not regularly arranged over a long-range are called amorphous materials. Example: Glass, rubbers.
Whereas semi-crystalline is the combination of both crystalline and amorphous materials.
2. What is the crystal structure of Austenitic stainless steel?
a) Face centered cubic structure
b) Body-centered cubic structure
c) Hexagonal close packed structure
d) Simple cubic structure
View Answer
Explanation: Austenitic stainless steel is an alloy of iron, chromium, and nickel. Due to the high content of chromium, the resultant stabilizes in the austenitic phase. Austenite usually exists in the FCC (Face centered cubic) crystal structure.
3. What is the crystal structure of Magnesium?
a) Face centered cubic structure
b) Body-centered cubic structure
c) Hexagonal close packed structure
d) Simple cubic structure
View Answer
Explanation: HCP is abbreviated as hexagonal close packed structure. Conditions for HCP is a1=a2≠a3 and α=β=60, γ̈=120, where a1, a2, a3 are unit vectors in three directions and α, β, γ̈ are angles between them. Magnesium possesses an HCP crystal structure.
4. Which of the following crystal structure is depicted in the given figure?
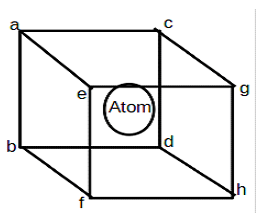
a) Face centered cubic structure
b) Hexagonal close packed structure
c) Body-centered cubic structure
d) Simple cubic structure
View Answer
Explanation: Simple cubic structure is a cube with lattice atoms only at the corners.
Face centered cubic structure is a cube with lattice atoms at the corners along with at the centers of cubic faces.
BCC is abbreviated as a Body-centered cubic structure. It is a cube with lattice atoms at the corner along with a single atom at the center of the cube.
5. Which of the following is/are the properties of metals?
a) Ductility
b) Electrical conductivity
c) Thermal conductivity
d) Ductility, electrical and thermal conductivity
View Answer
Explanation:
Ductility, malleability, conductors of heat and electricity, lustrous, definite melting and boiling point, lustrous, high strength, sonorous, etc are the properties of metals.
6. Which of the following types of bonds are non-directional in nature?
a) Covalent bond
b) Ionic bond
c) Metallic bond
d) Covalent and metallic bond
View Answer
Explanation: Non-directional bonding is a type in which each atom is bonded to many of its neighbors. Covalent and metallic bonds are non-directional in nature whereas ionic bond is directional in nature.
7. Grain boundaries are the mismatch regions formed between two grains during solidification.
a) True
b) False
View Answer
Explanation: Every metal microstructure consists of grains and grain boundaries. Grain boundaries are high energy areas, formed due to the mismatch between the grains during the solidification.
8. Which of the following is an example of a homogenous alloy?
a) 18-8 stainless steel
b) Low carbon steel
c) High carbon steel
d) Spheroidal cast iron
View Answer
Explanation: Homogenous alloy is the alloy in which the components are completely soluble in each other and it has only one phase. 18-8 stainless steel is a homogenous alloy with uniform composition and a single-phase structure.
9. Which of the following is an example of Heterogenous alloy?
a) High carbon steel
b) 18-8 stainless steel
c) Ni-Cu alloy
d) Bronze
View Answer
Explanation: Heterogenous alloy is the alloy in which the components are partially dissolved in each other and it has two or more phases. High carbon steel consists of alpha iron, cementite phase, and lamellar structure.
10. Grain boundaries are highly prone to corrosion than the grain faces.
a) True
b) False
View Answer
Explanation: Grain boundaries are the high energy area and are more active chemically. Hence, grain boundaries are highly prone to corrosion than the grain faces. This is used to develop a contrast between grain and grain boundaries in the inspection of microstructure.
11. Which of the following type of alloys are highly corrosion resistant in nature?
a) Homogenous alloys
b) Heterogenous alloys
c) Homogenous and heterogeneous alloys
d) Precipitation strengthened alloys
View Answer
Explanation: Homogenous alloys are highly corrosion resistant alloys than other alloy types. Since the galvanic effect is not present in homogenous alloys because of its single phase.
12. Which of the following conditions are applied to the orthorhombic crystal structure?
a) a1=a2=a3 and α=β=\(\ddot{\gamma}\)=90
b) a1=a2≠a3 and α=β=\(\ddot{\gamma}\)=90
c) a1≠a2≠a3 and α=β=\(\ddot{\gamma}\)=90
d) a1≠a2≠a3 and α=β=\(\ddot{\gamma}\)≠90
View Answer
Explanation: Crystal structures are divided into 14 different types based on the structure of the unit cell and arrangement of atoms in it. These 14 different types are called Bravais lattice. And the conditions for orthorhombic crystal structure is a1≠a2≠a3 and α=β=γ̈=90
, where a1, a2, a3 are unit vectors in three directions and α, β, γ̈ are angles between them.
13. Which of the following is the correct option regarding the packing efficiency of crystal structures?
a) FCC=HCP>BCC
b) FCC>HCP=BCC
c) FCC<BCC<HCP
d) HCP>FCC>BCC
View Answer
Explanation: Packing efficiency = (volume of atoms in unit cell/volume of the unit cell)*100. Packing-efficiency of FCC, HCP, and BCC is 74%, 74%, and 68%. Therefore, the correct order is FCC=HCP>BCC.
14. What is the ROI in economic consideration of corrosion?
a) Refund on investment
b) Return on investment
c) Refund on income
d) Return on income
View Answer
Explanation: ROI means Return on investment.
ROI=[(Oa+Ia/na)–(Ob+Ib/nb)/Ib–Ia]*100 where O = annual costs including maintenance cost, I=Investment, n=anticipated life in years, and subscripts a and b refer to the present and proposed installations, respectively.
15. What is NPV in economic consideration of corrosion?
a) Net profit volume
b) Net present value
c) Net profit value
d) Net precision value
View Answer
Explanation: NPV provides the most accurate basis for analyzing business costs and can be directly applied to the economics of corrosion control. But it needs extensive calculations.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering for Experienced people, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Mechanical Engineering Internship
- Apply for Metallurgical Engineering Internship
- Practice Mechanical Engineering MCQs
- Check Corrosion Engineering Books
- Check Metallurgical Engineering Books
