This set of Corrosion Engineering written test Questions & Answers focuses on “Environments – Liquid Metals and Fused Salts”.
1.The liquid-metal corrosion is usually a physical effect rather than an electrochemical attack.
a) True
b) False
View Answer
Explanation: Liquid metal corrosion is usually a physical effect rather than an electrochemical attack. Electrochemical attack includes reduction and oxidation of hydrogen ions and metallic atoms respectively.
2. Which of the following is/are the types of liquid-metal corrosion?
a) Solution of structural metal
b) Diffusion of liquid into solid metal
c) Intermetallic compound formation
d) Solution of structural metal, diffusion of liquid into solid metal and intermetallic compound formation
View Answer
Explanation: Types of liquid-metal corrosion are:
i. Solution of structural metal
ii. Diffusion of liquid into solid metal
iii. Intermetallic compound formation
iv. Mass transfer.
3. Which of the following is/are undesirable in heat-exchange systems handling liquid metals?
a) Deposition of impurities in cool areas
b) Resistance to heat transfer
c) Formation of brittle intermetallic compounds, resistance to heat transfer and deposition of impurities in cool areas
d) Formation of brittle intermetallic compounds
View Answer
Explanation: Conditions that are undesirable in heat-exchange systems handling liquid metals:
i. Deposition of impurities in cool areas
ii. Resistance to heat transfer
iii. Formation of brittle intermetallic compounds.
4. Which of the following parameter has a direct relation with the corrosion rate in liquid metals?
a) Percentage of alloying elements
b) Solubility of the metallic structure in liquid metal
c) Solubility of alloying elements in metallic matrix
d) Percentage of oxygen
View Answer
Explanation: Solubility of alloying elements in metallic matrix has direct relation with corrosion rate in liquid metals. Whereas liquid-metal corrosion is a physical effect rather than a chemical effect.
5. Which of the following parameter is taken as Xin the given graph regarding corrosion of various metals in liquid-metal corrosion?
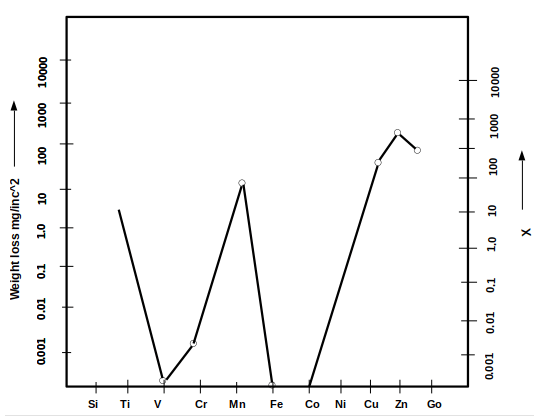
a) Atomic weight
b) Weight loss
c) Solubility percentage
d) Atomic number
View Answer
Explanation: The given graph shows the direct relation of corrosion rate with solubility of metallic structures in liquid mercury for various metals. And solubility of the metallic component in liquid mercury is taken as x.
6. Which of the following materials shows good resistance to both liquid-metals and high-temperature oxidation?
a) High alloy stainless steels
b) Monel
c) Hastelloy
d) Hastelloy, high alloy stainless steels and monel
View Answer
Explanation: Materials that show good resistance to both liquid-metals and high-temperature oxidation are 18Cr-8Ni stainless steel, high alloy stainless steel, monel, and Hastelloy.
7. Which of the following type of stainless steels are attacked by magnesium at its melting point?
a) Duplex stainless steels
b) Austenitic stainless steels
c) Martensitic stainless steels
d) Ferritic stainless steels
View Answer
Explanation: Austenitic stainless steels are the types of stainless steels that are attacked by magnesium at its melting. Liquid magnesium preferentially leaches out nickel from this alloy from the iron matrix.
8. The presence of traces of titanium and magnesium in mercury can inhibit the corrosion of iron in ferrous-based alloys.
a) True
b) False
View Answer
Explanation: Traces of titanium and magnesium in mercury can inhibit the corrosion of iron in ferrous-based alloys. And it is reliable to use carbon steel in mercury for up to 540°C.
9. Which of the following materials have superior corrosion resistance and high rupture strength in mercury?
a) 5% chromium steels
b) Si-Cr-Mo steels
c) 5% chromium steels and Si-Cr-Mo steels
d) Carbon steels
View Answer
Explanation: Now-a-days 5% chromium steels and Si-Cr-Mo steels replace carbon steels in mercury due to its superior corrosion resistance and high rupture strength.
10. Which of the following type of corrosion Is usually encounters for various materials in liquid metal environment?
a) Uniform corrosion
b) Stress cracking corrosion
c) Crevice corrosion
d) Pitting corrosion
View Answer
Explanation: Stress cracking corrosion is a type of corrosion that usually encountered in various materials in a liquid metal environment. This is due to the chemisorption of liquid-metal atoms and a reduction in tensile strength at the crack tip.
11. Which of the following alloys is/are usually used for sodium hydroxide fused salt?
a) Nickel-based alloys
b) Copper-based alloys
c) Ferrous alloys
d) Zinc based alloys
View Answer
Explanation: Sodium hydroxide is the most commonly used fused salt. And nickel-based alloys show good corrosion resistance to sodium hydroxide environment.
12. Which of the following is/are the types of mass transfer due to liquid-metal corrosion?
a) Composition-gradient mass transfer
b) Thermal-gradient mass transfer
c) Neither composition nor thermal gradient mass transfer
d) Both composition and thermal gradient mass transfer
View Answer
Explanation: Types of mass transfer due to liquid-metal corrosion are:
i. Composition-gradient mass transfer
ii. Thermal-gradient mass transfer.
13. Which of the following types of steel coatings is/are preferred for automobile body parts?
a) Galvanized steels
b) Electroplated steels
c) Galvanized steels, electroplated steels and complete immersion of corrosion preventing primer coat
d) Complete immersion of corrosion preventing primer coat
View Answer
Explanation: Types of steel coatings that are preferred for automobile parts are:
i. Galvanized steels
ii. Electroplated steels
iii. Complete immersion of corrosion preventing primer coat.
14. Which of the following is/are preventive measures to minimize automobile corrosion?
a) Use of inhibitor in the engine cooling system
b) Avoid very short runs
c) Fill the gasoline tank at least half full
d) Use of inhibitor in engine cooling system, avoid very short runs and fill the gasoline tank at least half full
View Answer
Explanation: Preventive measures to minimize automobile corrosion are:
i. Use of inhibitor in the engine cooling system
ii. Avoid very short runs
iii. Fill the gasoline tank at least half full.
To practice all written questions on Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Mechanical Engineering Internship
- Check Corrosion Engineering Books
- Practice Mechanical Engineering MCQs
- Practice Metallurgical Engineering MCQs
- Check Metallurgical Engineering Books
