This set of Corrosion Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Eight Forms – Galvanic Corrosion – 1”.
1.What is the other name of galvanic corrosion?
a) Bi-metallic corrosion
b) Mono-metallic corrosion
c) Localized corrosion
d) Mono-metallic and localized corrosion
View Answer
Explanation: Galvanic corrosion can be termed as bi-metallic corrosion or two-metal corrosion. This is because the driving force for current transfer and corrosion is the potential developed between the two metals.
2. Corrosion of less corrosion-resistant metal is usually increased and the attack of the more corrosion-resistant is decreased in galvanic corrosion.
a) True
b) False
View Answer
Explanation: As galvanic corrosion is a bi-metallic corrosion, relatively less corrosion-resistant metal preferentially acts as anode and relatively high corrosion-resistant metal preferentially acts as a cathode. This increases attack on the less corrosion-resistant metal (anode) and decreases on high corrosion-resistant metal (cathode).
3. Which of the following is the driving force in galvanic corrosion?
a) Conductivity of electrolyte
b) Crystal structure of metals
c) The potential difference between the two metals
d) Temperature of electrolyte
View Answer
Explanation: The principle driving force for current transfer and corrosion is the potential developed between the two metals. This potential difference can be altered by various factors such as conductivity of electrolyte and temperature of the electrolyte.
4. Which of the following will act as cathode and anode respectively in a dry-cell battery?
a) Zinc and carbon
b) Carbon and zinc
c) Magnesium and zinc
d) Zinc and ammonium chloride
View Answer
Explanation: Battery is a combination of cells in which chemical energy is converted into electrical energy. Carbon (noble electrode) acts as cathode and zinc (active electrode) acts as an anode. Moist ammonium chloride is used as an electrolyte.
5. Which of the following is the primary characteristic of an electrolyte to form corrosion?
a) Electrical resistivity
b) Thermal resistivity
c) Thermal conductivity
d) Electrical conductivity
View Answer
Explanation: Electrical conductivity is the primary characteristic of an electrolyte. Electrolyte is a medium to transfer electrons from anode to cathode, this results in corrosion. The conductivity of the electrolyte is one of the main factors in the regulation of corrosion.
6. What are the conditions to measure the standard reduction potential of metals against SHE?
a) 2 atm pressure, 25°C temperature, 2M concentration of H+
b) 2 atm pressure, 25°C temperature, 1M concentration of H+
c) 1 atm pressure, 25°C temperature, 1M concentration of H+
d) 1 atm pressure, 25°C temperature, 2M concentration of H+
View Answer
Explanation: The conditions to measure the standard reduction potential of metal electrodes against standard hydrogen electrode (SHE) are 1 atmospheric pressure, 25°C temperature and 1 molarity concentration of H+ ions.
7. What is the standard reduction potential value of gold in the EMF series?
a) +1.498 V
b) +1.2 V
c) +0.987 V
d) +0.799 V
View Answer
Explanation: Standard reduction potential values of metal are measured against SHE.
Standard reduction potential of gold (Au-Au+3), platinum (Pt-Pt+2), palladium (Pd-Pd+2), silver (Ag-Ag+1) are +1.498V, +1.2V, +0.987V and +0.799V respectively.
8. What is the standard reduction potential value of potassium in the EMF series?
a) +0.337 V
b) -2.925 V
c) -0.763 V
d) -1.662 V
View Answer
Explanation: Standard reduction potential values of metal are measured against SHE, which is arbitrarily defined as zero. Standard reduction potential of potassium (K-K+), aluminum (Al-Al+3), zinc (Zn-Zn+2), copper (Cu-Cu+2) are –2.925V, -1.662V, -0.763V and +0.337V respectively.
9. What is the abbreviation of EMF?
a) Electromagnetic force
b) Electromotive frequency
c) Electromotive force
d) Electrode motive force
View Answer
Explanation: EMF is abbreviated as an electromotive force. It is defined as the potential difference between two points (cathode and anode) in a circuit. It is the cause of the flow of current in the circuit.
10. Which of the following electrolyte is used for the preparation of a general galvanic series?
a) Polluted seawater
b) Unpolluted river water
c) Polluted river water
d) Unpovlluted seawater
View Answer
Explanation: Galvanic series is the most accurate prediction of galvanic relationships of metals. It is based on potential measurements and galvanic corrosion tests in unpolluted seawater. This includes metals and alloys along with their active and passive states.
11. Which of the following metal combination has a minimum galvanic effect?
a) Monel and copper
b) Inconel (passive) and Inconel (active)
c) 18-8 stainless steel (active) and 18-8 stainless steel (passive)
d) Titanium and lead
View Answer
Explanation: The galvanic effect can be reduced by selecting close metal combinations. The position of a metal or an alloy in galvanic is mainly affected by its composition and its state (active or passive). Monel (70 Ni, 30Cu) and copper is the close metal combination, hence it has a minimum galvanic effect.
12. What does the square bracket [] in the galvanic series indicates?
a) Most practical metal combinations
b) High corrosion-resistant metals
c) Low corrosion-resistant metals
d) Avoidable metal combinations
View Answer
Explanation: The bracket in the galvanic series indicates the most practical metal combinations. This metal combination results in little danger of galvanic corrosion.
Example: [ Monel, bronzes, copper, brasses]
13. The potential generated by two dissimilar metal combinations can change with time.
a) True
b) False
View Answer
Explanation: The potential developed between two dissimilar metals is usually decreases. This is due to the accumulation of reaction or corrosion products at the anode or cathode. This results in a reduction of corrosion kinetics.
14. Which of the following is the most corrosion-resistant metal at room temperature?
a) Titanium
b) Platinum
c) Gold
d) Tantalum
View Answer
Explanation: Tantalum is the most corrosion-resistant metal at room temperature. It is a rare, hard and lustrous transition metal. It is denoted by Ta and its atomic number (z) is 73.
15. Which of the following corrosion is depicted in the given figure?
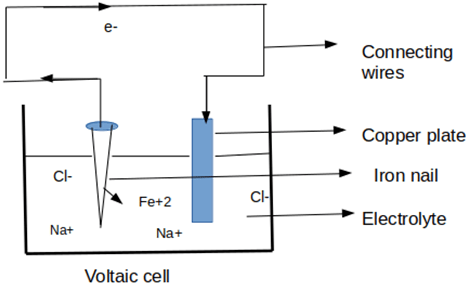
a) Crevice corrosion
b) Pitting corrosion
c) Galvanic corrosion
d) Uniform corrosion
View Answer
Explanation: Voltaic cell is the cell in which chemical energy is converted into electrical energy by participating in redox reactions. In this cell, preferential cathode and anode are formed and the galvanic effect is observed. Thus, galvanic corrosion is depicted in the given figure.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Mechanical Engineering MCQs
- Apply for Mechanical Engineering Internship
- Check Mechanical Engineering Books
- Check Metallurgical Engineering Books
- Check Corrosion Engineering Books
