This set of Corrosion Engineering Interview Questions and Answers focuses on “Corrosion and its Classification – 2”.
1. Which of the following factors affect the corrosion rate of metal?
a) Relative surface area of an anode and cathode
b) Nature of the oxide layer
c) Purity of metal
d) Relative surface area of an anode and cathode, nature of oxide layer and purity of metal
View Answer
Explanation: As the cathodic to anodic area increases, the more oxygen reduction, or more corrosion can occur. The corrosion resistance of aluminum can be attributed to the formation of the thick, dense oxide layer. Impure metals undergo corrosion at a faster rate than the pure metals.
2. The cathodic reaction that occurs during corrosion in oxygenated acidic solution is ______
a) 2H++2e–==>H2
b) 4H++O2+4e–==>2H2O
c) 2H2O+2e–==>H2+2OH–
d) 2H2O+O2+4e–==>4OH–
View Answer
Explanation: The common hydrogen reduction reaction in corrosion will proceed based on the pH and the amount of oxygen present in the solution. Then oxygenated acidic solution (pH < 7) will react as
4H++O2+4e–==>2H2O.
3. If a metal undergoes uniform corrosion it becomes _____
a) thinner
b) thicker
c) perforated
d) thicker and perforated
View Answer
Explanation: Uniform corrosion is the most common form of corrosion. It is characterized by an electrochemical reaction that proceeds uniformly over the entire exposed surface. Thus, the metal becomes thinner.
4. Which theory explains the oxidation of metals?
a) Collison theory
b) Molecular orbital theory
c) Wagner’s theory
d) Mixed potential theory
View Answer
Explanation: Wagner’s theory states that the oxidation of metal is electrolytic in nature and the rate of oxidation is determined by the electrical properties of the corrosion product.
5. What is the mechanism of dry corrosion?
a) Absorption
b) Electrochemical
c) Differential solubility
d) Both electrochemical and differential solubility
View Answer
Explanation: Dry corrosion occurs in the absence of a liquid phase. Vapors and gases are usually the corrodents. These will hit and adsorbed on the metal surface to form corrosion. So dry corrosion can be explained by an absorption mechanism.
6. Which of the following corrosion contaminant will act as a protective layer?
a) AgCl
b) SnCl2
c) Fe2O3
d) CaO
View Answer
Explanation: Silver on reaction with chlorine forms silver chloride. It acts as a protective layer and prevents chlorine to corrode further. Here SnCl2 and CaO are volatile in nature whereas Fe2O3 is porous in nature. Hence, they can’t act as protective layers.
7. Corrosion of iron takes place even in the solid ice.
a) True
b) False
View Answer
Explanation: Though ice is the solid form of water but it doesn’t conduct electrons. The function of an electrolyte is to transfers ions from one electrode to another. Thus, given statement is false. Corrosion needs an anode, a cathode and a conductive electrolyte.
8. What are the factors that affect corrosion?
a) Temperature
b) Humidity
c) Conductance of the corroding medium
d) Temperature, humidity and conductance of the corroding medium
View Answer
Explanation: The main factors that affect the rate of corrosion includes the effect of pH, temperature, humidity, conductance of the corroding medium, oxygen concentration, presence of impurities. These factors are based on the nature of environment.
9. _______ is a chemical substance which reduces the corrosion rate.
a) Corrosion inhibitor
b) Corrosion initiator
c) Electrolyte
d) Corrosive medium
View Answer
Explanation: An inhibitor is a substance that, when added in small concentrations to an environment, decreases the corrosion rate.
Following are the types of inhibitors:
i. Adsorption-type
ii. Hydrogen-evolution poisons
iii. Scavengers
iv. Vapor-phase
10. In the given figure, what does point A denote?
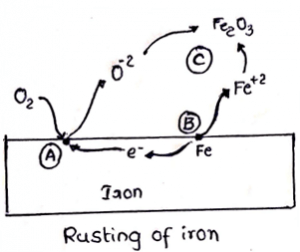
a) Cathodic spot
b) Anodic spot
c) Electrolyte
d) Oxide layer
View Answer
Explanation: Cathodic spot, a place on the metal surface where the reduction reaction takes place. Reduction is a process in which atoms of the chemical reaction accepts electrons in order to form anion.
11. In the given figure, what does point B denote?

a) Cathodic spot
b) Anodic spot
c) Electrolyte
d) Oxide layer
View Answer
Explanation: Anodic spot, a place on the metal surface where the oxidation reaction takes places. Oxidation is a loss of electrons or increase in the oxidation state during a reaction by a molecule or atom. Molecules or atoms on oxidation forms cations. Ex: Fe (oxidation state is 0) ==> Fe+2 (oxidation state is 2) + 2e–.
12. In the given figure, what does point C denote?

a) Cathodic spot
b) Anodic spot
c) Electrolyte
d) Oxide layer
View Answer
Explanation: Electrolyte is a conductive medium between anode and cathode. It undergoes ionization when dissolved in water or ionizing solvents. The function of an electrolyte is to transfers ions from one electrode to another.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering for Interviews, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Mechanical Engineering Internship
- Practice Mechanical Engineering MCQs
- Apply for Metallurgical Engineering Internship
- Practice Metallurgical Engineering MCQs
- Check Metallurgical Engineering Books
