This set of Corrosion Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Corrosion Principles – Electrochemical Aspects – 1”.
1. Which of the following products are obtained when a zinc metal is dipped in dilute HCl solution?
a) H2, ZnCl2
b) Cl2, ZnH2
c) H2, Cl2 and ZnH2
d) Zn, H2 and Cl2
View Answer
Explanation: When zinc is placed in dilute hydrochloric acid, a vigorous reaction occurs. Hydrogen gas is evolved and the zinc dissolves, by forming a solution of zinc chloride.
Anodic reaction: Zn==>Zn+2+2e(Zn+2+2Cl– ==>ZnCl2)
Cathodic reaction: 2H++2e==>H2
2. Which of the following reactions occurs when iron is immersed in (oxygenated) sea water?
a) Fe==>Fe+2+2e, 2H++2e==>H2
b) Fe==>Fe+2+2e, O2+2H2O+4e==>4OH–
c) Fe+2+2e==>Fe+2, 4H++O2+4e==>2H2O
d) Fe+2+2e==>Fe+2, 2H++2e==>H2
View Answer
Explanation: Iron dissolution (anodic reaction) Fe==>Fe+2+2e. Oxygenated hydrogen reaction (cathodic reaction) in a neutral solution (pH=7) is O2+2H2O+4e==>4OH–.
3. Which of the following is more stable form of iron in an oxygenated solution?
a) Fe2O3
b) Fe(OH)2
c) 2Fe(OH)3
d) Fe3O4
View Answer
Explanation: +3 oxidation state of iron is more stable state of iron than +2 oxidation state. The reaction is follows as
Fe+H2O+½O2==>Fe(OH)2
2Fe(OH)2+H2O+1/2O2==>2Fe(OH)3.
4. Which of the following are the types of chemical reactions?
a) Combination and Decomposition reactions
b) Combination and Single displacement
c) Single and Double displacement reaction
d) Combination, Decomposition, Single displacement and double displacement
View Answer
Explanation: Combination reaction: A+B==>C,
Decomposition reaction: A==>B+C,
Single displacement reaction: AB+C==>AC+B
Double displacement reaction: AB+CD==>AC+BD
5. More than one anodic and cathodic reactions are possible in corrosion.
a) True
b) False
View Answer
Explanation: Mixed potential theory states that any electrochemical reaction can be divided into two or more partial reactions (anodic and cathodic reactions). Dissolution of an alloy in pure acid involves more than one anodic reaction. Corrosion of pure metal in oxygenated impure acid involves more than one cathodic reaction.
6. Oxygenated acids are more corrosive than oxygen-free acids.
a) True
b) False
View Answer
Explanation: Case1 (Oxygen-free acids)
Anodic reaction: M==M>+n+ne
Cathodic reaction: 2H++2e==>H2
Case2 (Oxygenated acids)
Anodic reaction: M==>M+n+ne
Cathodic reaction: 2H++2e==>H2, O2+4H++4e==>2H2O
This oxygen reduction increases the cathodic rate which correspondingly increases the anodic rate or corrosion rate.
7. Which of the following parameter of electrolyte decreases the corrosion rate?
a) Dissolved oxygen
b) Temperature
c) High electrical resistance
d) Presence of ferric ions
View Answer
Explanation: The Function of an electrolyte is to transfer the ions between anode and cathode. Increase in electrical resistance in electrolyte shows obstruction for the free flow of ions, hence the corrosion rate decreases. Whereas dissolved oxygen, temperature and presence of ferric ions increases the mobility of ions and increases the corrosion rate.
8. Which of the following are the types of polarization?
a) Activation polarization
b) Activation and Concentration polarization
c) Resistance polarization
d) Activation, Concentration and Resistance polarization
View Answer
Explanation: Polarization is the potential difference occurred due to the non-equilibrium conditions, due to the change in concentration or temperature. These are classified into three types. They are i) Activation polarization ii) Concentration polarization iii)Resistance polarization.
9. Which of the following is depicted in the given figure?
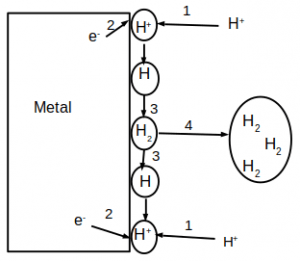
a) Activation polarization
b) Concentration polarization
c) Resistance polarization
d) Concentration and Resistance polarization
View Answer
Explanation: Activation polarization refers to an electrochemical process that is controlled by the reaction sequence at the metal-electrolyte interface.
Step 1: Species adsorbed on the metal surface
Step 2: Electron transfer
Step 3: Reduction of species
Step 4: Combine to form a bubble of hydrogen molecule.
10. Which of the following is depicted in the given figure?
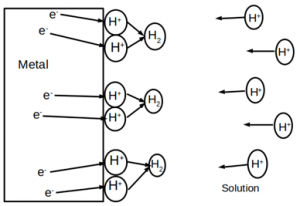
a) Activation polarization
b) Concentration polarization
c) Resistance polarization
d) Both Activation and Resistance polarization
View Answer
Explanation: Concentration polarization refers to an electrochemical process that is controlled by the diffusion of ions from bulk solution to the metal-electrolyte interface. This usually occurs in low concentration electrolytes.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Mechanical Engineering MCQs
- Practice Metallurgical Engineering MCQs
- Check Metallurgical Engineering Books
- Check Corrosion Engineering Books
- Apply for Mechanical Engineering Internship
